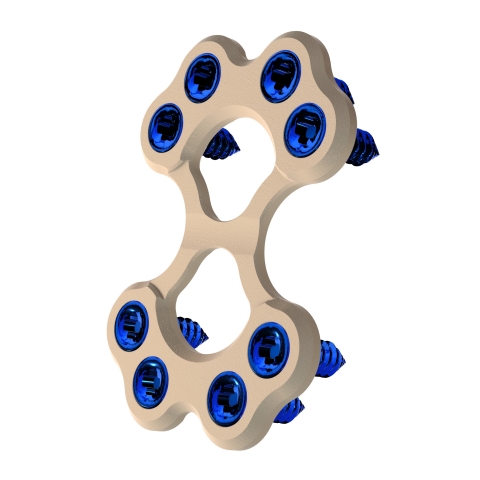
MARQUETTE, Mich.--(BUSINESS WIRE)--Able Medical Devices, Inc. (Able) today announced the U.S. FDA 510(k) clearance of the Valkyrie™ Thoracic Fixation System. Valkyrie is intended for use in the stabilization and fixation of fractures of the chest wall, including sternal fixation following sternotomy, as well as sternal reconstructive surgical procedures for patients with normal and/or poor bone.
“The Valkyrie system features the clinically proven performance of rigid fixation combined with the use of novel materials and the benefits of bioactive science to maximize sternal stability and promote bone healing,” said Peter J. Didyk, VP & managing director of cardiothoracic and an inventor of the Valkyrie system. “Its patient-specific design offers intraoperative choices, and its minimal instrumentation focuses on speed, efficiency, and simplicity in the O.R., making it a highly desirable product on the market.”
The breakthrough PEEK-based system can easily be cut during emergent re-entry, prevents post-op image scatter and conforms to meet various patient anatomies. The double lead screws insert into the bone at a faster rate and increase bone purchase, while their HAnano surface treatment recruits cellular adhesion and quickly synthesizes bone to create a more rigid closure.
“Surgeons may choose from an array of various implant shapes and sizes, but after a long case, the simplicity of the system removes much of the guesswork which is greatly preferred,” said Steven J. Hicks, CEO & president of parent company J.M. Longyear. “This sterile-packaged, single instrument system offers a wide variety of benefits while its bioactive screws and contourable plates set an industry standard in sternal closure.”
The Valkyrie system delivers both clinical and economic value to its consumers. Its ability to individualize itself to each specific patient and its single use design reduces waste to the healthcare system while maximizing patient benefits.
About Able Medical
Able Medical Devices is a leader in contract manufacturing and development. Able partners with companies seeking first class, outsourced services from design & development to comprehensive finished goods manufacturing. The company’s extensive expertise includes working with surgeons and OEMs in spine, trauma and cardiothoracic markets. Able specializes in developing full surgical systems as well as custom instrumentation. For more information, see ablemedicaldevices.com.