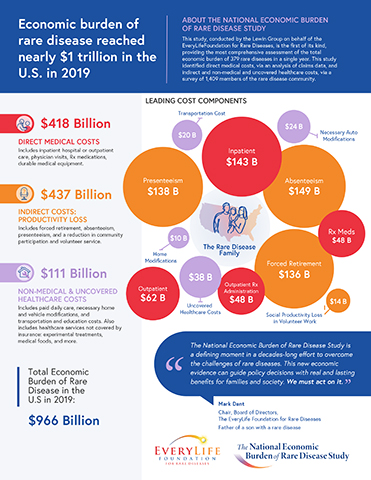
WASHINGTON--(BUSINESS WIRE)--In a new study released today by the EveryLife Foundation for Rare Diseases, the estimated economic cost of 379 rare diseases in the United States totaled $966 billion in 2019, a figure that surpasses the economic burden estimated for many of the costliest chronic diseases described by the Centers for Disease Control and Prevention (CDC), including diabetes, heart disease, and cancer.
The estimate is one of the main conclusions of The National Economic Burden of Rare Disease Study, the most comprehensive assessment of the total cost of rare diseases in the U.S. to date. Top-line results from the study were presented at a briefing today hosted by the Rare Disease Congressional Caucus to highlight the devastating economic impact of rare diseases on patients, families, caregivers, employers, and society. The study authors plan to submit the results for publication.
“This landmark report changes the conversation about rare disease by moving from back-of-the-envelope estimates to quantifiable data that reflect the true, massive economic impact a diagnosis has on families in the U.S. rare disease community,” said Annie Kennedy, chief of policy and advocacy at the EveryLife Foundation. “Based on these data, rare diseases represent an urgent public health crisis that demands additional research, enhanced awareness, and improved access to diagnosis, care, and treatment.”
Direct Medical Costs Totaled $418 Billion in 2019
In the U.S., a disease is defined as rare when it affects fewer than 200,000 people. While there are estimated to be more than 7,000 rare diseases, The National Economic Burden of Rare Disease Study includes 379 rare diseases in its assessment. The study estimates that individuals affected by these diseases incurred direct medical costs amounting to $418 billion in 2019, including expenditures for inpatient hospital or outpatient care, physician visits, prescription medications, and durable medical equipment.
Indirect and Non-Medical Costs Totaled $548 Billion in 2019
The study also identifies indirect and non-medical costs, estimated at an additional $548 billion in 2019, as the primary cost drivers of the rare disease economic burden in the U.S. This estimate sheds light on a set of costs that previous studies did not examine across rare diseases. It includes the indirect costs of lost productivity due to forced retirement, absenteeism, “presenteeism” (lost productivity occurring when employees cannot fully function in the workplace), and social productivity loss in community participation and volunteer work.
This estimate also encompasses costs of healthcare services not covered by insurance, including experimental treatments, alternative or non-traditional treatments such as acupuncture, as well as medical foods and dental surgeries. Non-medical costs include paid daily care, necessary home and vehicle modifications, transportation costs, home schooling, missed schooling, and special education.
“This research reveals so much more than dollars and cents. It brings to life the overwhelming and unrelenting financial, medical, and emotional challenges of living with a rare disease,” commented Marissa Penrod, mother of a son with a rare disease, founder of Team Joseph, and an advisor to the study team. “For many families, the journey begins with the search for a diagnosis and hope for an effective treatment. It very quickly evolves into navigating a complex medical system, engaging in battles with insurance companies, and paying out-of-pocket for necessary home and vehicle modifications. This all happens within the typical demands of a family, while trying to hold down a job and maintain some quality of life. This report should be a call to action. We can do more to help our families, and we must.”
Study Methodology
As part of the methodology of the study, researchers combined the prevalence of rare diseases with per-capita costs to derive the national economic burden. To estimate prevalence and the direct medical cost, the study evaluated claims data from three sources: Medicare, Medicaid, and the Optum de-identified Normative Health Information System, a large and geographically diverse claims database for the privately insured.
The study also included a primary survey designed to deepen understanding of the full spectrum of the impact of rare disease in the U.S. The survey collected detailed data on a broad set of indirect and non-medical costs of rare disease that were previously unavailable, especially the impact of rare disease on unpaid caregivers. One of the largest surveys thus far of multiple rare disease communities, the survey generated 1,399 fully completed responses from individuals representing about 400 rare disease communities.
“The data from this study provide a more complete picture of the medical burdens of rare disease patients in the United States,” said Anne Pariser, M.D., director of the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences (NCATS), which is part of the National Institutes of Health. “We will be using these data to help guide ongoing efforts to improve diagnosis and identify additional areas for research prioritization to benefit the rare disease community,” added Dr. Pariser, who is an advisor to the study.
Study Contributors
The EveryLife Foundation, the study’s sponsor, commissioned the Lewin Group to estimate the economic impact of rare disease in the U.S. in 2019, and assembled a distinguished steering committee of technical advisors and expert contributors representing government, industry, academia, and rare disease communities. The study secured funding support from Alexion Pharmaceuticals, Amicus Therapeutics, Argenx US, Inc., AVROBIO, Chiesi Global Rare Diseases, Enzyvant Therapeutics, Genentech, Mallinckrodt Pharmaceuticals, PhRMA, Pfizer Inc., REGENXBIO Inc., Sanofi Genzyme, Sarepta Therapeutics, Spark Therapeutics, and Travere Therapeutics, Inc.
“By conducting a thorough analysis of medical databases, combined with primary research within rare disease communities, we were able to address a significant knowledge gap and quantify the economic toll of rare diseases in the U.S.,” noted Grace Yang, MPA, MA, vice president at the Lewin Group. “We are grateful to the thousands of community members who made the study possible, as well as the EveryLife Foundation and the study steering committee.”
Study Limitations
The report estimates that 15.5 million individuals in the U.S. have any of the 379 rare diseases included in the study. Those diseases constitute a subset of the nearly 7,000 rare diseases that have been identified worldwide. The study authors caution that their economic impact estimate represents a conservative estimate and is not generalizable to all rare diseases. Other limitations of the study include reliance on one diagnosis code to estimate direct medical costs of a disease matched to that code; limited availability of International Classification of Diseases (ICD) codes for certain rare diseases; reliance on self-reported data to estimate indirect and non-medical costs; and small sample sizes, which prevented the researchers from breaking down burden estimates by desired population data, such as by sex or by race/ethnicity. Despite those limitations, the study is the largest and most comprehensive effort thus far in the U.S. to measure the societal impact of many rare diseases at once.
For more information about The National Economic Burden of Rare Disease Study, visit the study website at burdenstudy.org or the EveryLife Foundation for Rare Diseases website at everylifefoundation.org.
About the EveryLife Foundation
The EveryLife Foundation for Rare Diseases is a 501c(3) nonprofit, nonpartisan organization dedicated to empowering the rare disease patient community to advocate for impactful, science-driven legislation and policy that advances the equitable development of and access to lifesaving diagnoses, treatments, and cures. The Foundation provides the training, education, resources and opportunities to make patient voices heard, help change public policy and save lives.
About the Lewin Group
For more than 50 years, the Lewin Group has provided health care and human services policy research, analytics, and consulting to federal and state clients. Lewin is part of OptumServe and the UnitedHealth Group family of companies.