SANTA CLARA, Calif.--(BUSINESS WIRE)--Ancora Heart, Inc., a company developing a novel therapy to address heart failure, today announced enrollment of the first patient in the CORCINCH-HF pivotal trial, which is designed to evaluate the safety and efficacy of the AccuCinch® Ventricular Restoration System in patients who have symptomatic heart failure (HF) with reduced ejection fraction (HFrEF). This study is being conducted to support the submission of a Premarket Approval (PMA) application to the U.S. Food and Drug Administration (FDA).
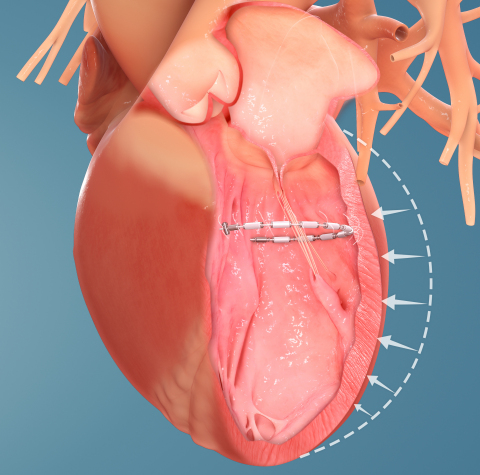
As the only completely transcatheter procedure to treat the enlarged left ventricle, the AccuCinch System is a fundamentally different and innovative device-based therapy designed to improve the structure and function of the heart and help bring relief to HF patients who remain symptomatic despite current guideline-directed medical care.
“In patients with heart failure, the left ventricle often becomes enlarged as it has to work harder than a healthy heart to pump blood throughout the body,” said Ulrich Jorde, MD, global co-principal investigator of the CORCINCH-HF Study; professor of medicine, Albert Einstein College of Medicine; and section head of Heart Failure, Cardiac Transplantation and Mechanical Circulatory Support at Montefiore Health System in New York.1 “By providing structural support to the ventricle with the AccuCinch System, we aim to evaluate if the ventricle will be able to pump more efficiently and help to reduce the symptoms of the disease in these patients.”
The CORCINCH-HF Study is a prospective, randomized, open-label, multicenter, international, clinical safety and efficacy investigation of the AccuCinch System, which is designed to enroll 400 patients at up to 80 centers worldwide. The study has a unique design allowing initial analysis of safety and clinical efficacy for PMA submission after the first 250 patients have reached six months of follow-up, and then a second analysis after the entire cohort has reached 12 months of follow-up. (ClinicalTrials.gov Identifier: NCT04331769)
The AccuCinch System is designed to augment the existing care cardiologists provide their HF patients. For patients in whom HF has progressed beyond the ability of medications and pacemakers to manage symptoms, the AccuCinch System may provide an effective treatment option by filling the gap between medication or pacemaker therapy and left ventricular assist devices (LVADs) or a heart transplant.
“The ability to offer additional treatment options to heart failure patients whose disease has progressed to the point that optimal medical therapy is no longer able to manage the symptoms is imperative,” said Jason R. Foerst, MD, medical director of the Carilion Clinic Structural Heart and Valve Program and assistant professor of medicine at Virginia Tech Carilion School of Medicine, whose team enrolled the first patient in the CORCINCH-HF Study. “My team and I are excited to be a part of the CORCINCH-HF Study and look forward to working with Ancora Heart to fulfill our role in this very important clinical trial.”
During the minimally invasive AccuCinch System procedure, a flexible implant is attached to the inner wall of the left ventricle and then cinched. The implant is intended to reduce the size of the left ventricle, reduce ventricular wall stress, and support and strengthen the heart wall. The AccuCinch System may enable improved functional capacity and quality of life for patients, and potentially slow or reverse the left ventricular remodeling associated with the progression of heart failure.
“We look forward to working with our clinical partners to conduct the CORCINCH-HF Study and together move closer to improving treatment options for the millions of people suffering from heart failure,” said Jeff Closs, president and CEO of Ancora Heart. “Previously collected data on early feasibility implants suggests that the AccuCinch System may afford significant clinical benefits to patients suffering from heart failure with reduced ejection fraction. We anticipate that the data from this study will confirm and expand upon these early findings and enable us to ultimately provide an important innovation that may halt or even reverse the progression of heart failure in many patients.”
About Heart Failure
An estimated 6.5 million U.S. adults live with heart failure, a condition in which the heart’s muscles weaken and lose their ability to pump enough oxygen-rich blood to the body.2 Heart failure patients suffer from debilitating symptoms including persistent exhaustion, trouble breathing, confusion and loss of memory. About half of HF patients have heart failure with a reduced ejection fraction (HFrEF) and an enlarged left ventricle, the main pumping chamber of the heart, which causes more stress on the heart and leads to reduced pumping efficiency. Up to 50 percent of people who develop heart failure die within five years of diagnosis.3
About Ancora Heart
Ancora Heart is a medical device company dedicated to providing new treatment options for people with heart failure (HF). The company’s proprietary AccuCinch® Ventricular Restoration System is the only completely transcatheter device designed to restore the structure and function of the enlarged left ventricle of the heart, thereby addressing the fundamental issue in the progression of heart failure in patients with reduced ejection fraction (HFrEF). The AccuCinch System is an investigational device, which is currently being evaluated in multiple clinical trials in the U.S. and E.U. Ancora Heart is a privately held company located in Santa Clara, Calif. For more information, please visit www.ancoraheart.com and connect on Twitter (@AncoraHeart) and LinkedIn (https://www.linkedin.com/company/24214239/).
_______________________
- Dr. Jorde received compensation from Ancora Heart for a speaking engagement in 2020.
- Murphy S, Ibrahim N, Januzzi J. Heart Failure with Reduced Ejection Fraction, A Review. JAMA. 2020;324(5):488-504
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-596.