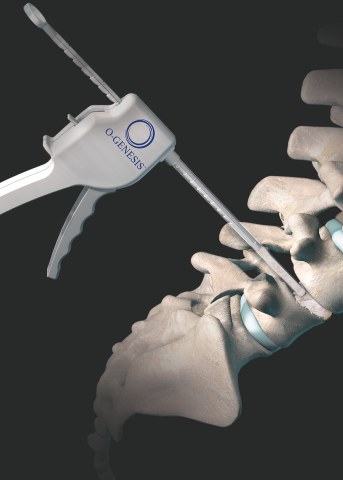
LEWISVILLE, Texas--(BUSINESS WIRE)--Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and extremities focus, today announced the launch of its new O-GENESIS™ Graft Delivery System during the North American Spine Society (NASS) 2020 virtual annual meeting. A complete bone graft delivery system, the O-GENESIS Graft Delivery System is designed to deliver allograft, autograft or synthetic bone graft to orthopedic surgical sites. The company is also introducing the AlloQuent™ Structural Allograft Q-Pack®, a hydrated, ready-to-use form of cervical and lumbar spacers for allograft procedures.
“Orthofix is committed to continuous technology innovation and expanding solutions to improve operating room efficiencies,” said Kevin Kenny, Global President of Orthofix Spine. “The unique design of the O-GENESIS Graft Delivery System enables one-handed delivery of bone graft material in a precise and controlled manner to aid surgeons with the targeted placement of biologics during orthopedic surgeries. Additionally, we are pleased to offer the new AlloQuent Structural Allograft in Q-Pack that allows for immediate use with no downtime for rehydrating. Both of these demonstrate our commitment to providing surgeons with easy-to-use solutions to meet the needs of their patients.”
The O-GENESIS Graft Delivery System works hand-in-hand with our flagship biologic solution, the Trinity ELITE™ Allograft with viable cells, processed exclusively for Orthofix by MTF Biologics. Designed with ergonomics in mind, the system enables single-handed delivery and features two streamlined loading options to accommodate a versatile choice of other bone graft materials, such as fiberFUSE™ Demineralized Bone Matrix.*
The AlloQuent Structural Allografts are precision-machined cervical and lumbar spacers. Orthofix has partnered with MTF Biologics to launch AlloQuent in the new Q-Pack Technology − a zero rehydration technology designed to help eliminate preparation time while preserving the mechanical properties of the allograft. AlloQuent structural allografts offer procedure specific spacers with surgical efficiency in ready-to-use packaging.
*Data on file with Orthofix Medical.
About Orthofix
Orthofix Medical Inc. is a global medical device and biologics company with a spine and extremities focus. The Company’s mission is to deliver innovative, quality-driven solutions as we partner with health care professionals on improving patients’ lives. Headquartered in Lewisville, Texas, Orthofix’s products are distributed in more than 70 countries via the Company’s sales representatives and distributors. For more information, please visit www.Orthofix.com.
Forward-Looking Statements
This communication contains certain forward-looking statements under the Private Securities Litigation Reform Act of 1995. These forward-looking statements, which may include, but are not limited to, statements concerning the estimates, projections, financial condition, results of operations and businesses of Orthofix and its subsidiaries, are based on Orthofix management’s current expectations and estimates and involve risks and uncertainties that could cause actual results or outcomes to differ materially from those contemplated by the forward-looking statements.
The forward-looking statements in this release do not constitute guarantees or promises of future performance. Factors that could cause or contribute to such differences may include, but are not limited to, risks relating to: practices of health insurance companies and other third-party payors with respect to reimbursement for our devices and other risks described in the “Risk Factors” sections of our 2019 Annual Report on Form 10-K and our Quarterly Reports on Form 10-Q for the periods ended March 31, 2020 and June 30, 2020. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. The Company undertakes no obligation to update or revise the information contained in this press release.