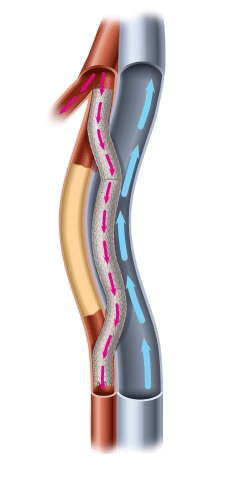
MILPITAS, Calif.--(BUSINESS WIRE)--PQ Bypass Inc, a medical device pioneer bringing new advancements to the treatment of advanced peripheral artery disease (PAD), announced today that they received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for the Detour System. The Detour System is the world’s first fully-percutaneous femoral-popliteal bypass device intended to treat extremely long, complex blockages in the superficial femoral artery (SFA).
Physician access to this device can now be expedited as a result of this designation by the FDA Breakthrough Device Program, which is intended to help patients receive more timely access to breakthrough technologies that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions. Under the program, the FDA will provide PQ Bypass with priority review and interactive communication regarding device development and clinical trial protocols, during the premarket review process.
“As this is a first-of-its-kind device, we are pleased to have the FDA recognize the novelty and potential of our therapeutic approach in treating severe SFA,” said Heather Simonsen, General Manager at PQ Bypass. “We appreciate their collaborative review of our Breakthrough Device designation request and look forward to a continued productive relationship as we continue towards PMA submission.”
The Detour System is designed to treat patients who are unable to carry out activities of daily living and are unable to enjoy the liberties of free mobility as a result of their advanced symptomology, severe lesion morphology, and multiple co-morbidities. Once PAD progresses to debilitating claudication or tissue loss, revascularization becomes imperative to mitigate the ongoing deterioration and to prevent amputation. Left untreated, these patients are at greater risk for further functional deterioration, major adverse limb events, and mortality.
“This designation is a major milestone for PQ Bypass, and we expect this technology to change the paradigm for complex SFA treatment in the way EVAR and TAVR changed the paradigm for aortic repair,” said Rich Ferrari, Chairman and CEO of PQ Bypass. “We are nearing enrollment completion in our DETOUR2 and TORUS2 IDE studies, and had new positive data on our technology published in the Journal of Vascular Surgery, making 2020 a momentous year for the company. This is an exciting time for all of us at PQ, with this important designation a credit to such a talented group. We are honored and proud to be part of this important journey to develop a first-line therapy in the treatment of advanced PAD.”
About Peripheral Arterial Disease:
According to the U.S. Centers for Disease Control and Prevention (CDC), approximately 18 million people in the United States suffer from PAD, a common circulatory problem in which narrowed arteries reduce blood flow to the limbs. Estimates suggest that anywhere from 12 to 20 percent of individuals over the age of 60 are living with PAD.
About PQ Bypass:
PQ Bypass Inc. is a medical device pioneer bringing new advancements to the treatment of advanced peripheral artery disease (PAD). Operated by recognized leaders in the medical device industry, including veterans from Boston Scientific, Philips, Medtronic, Abbott and Johnson & Johnson, PQ Bypass devices are built upon nearly a decade of research and patented technological advancement. The company’s physician-driven innovations include the Detour procedure for percutaneous femoropopliteal bypass and the proprietary TORUS stent graft, the first new stent graft developed for use in the SFA in 15 years.
PQ Bypass is currently sponsoring two multicenter IDE trials, DETOUR2 and TORUS2, to evaluate the safety and effectiveness of its technology platform in two distinct patient populations and build upon the body of clinical evidence collected in the DETOUR1 and TORUS1 studies.
For more information on DETOUR2, please visit https://clinicaltrials.gov/ct2/show/NCT03119233
For more information on TORUS2, please visit https://clinicaltrials.gov/ct2/show/NCT04130737
PQ Bypass is recognized by MedTech Outlook magazine as one of the Top 10 Cardiovascular Companies of 2019 and earned Frost and Sullivan’s European Technology Innovation award in 2017. The DETOUR system and the TORUS Stent Graft are limited by federal law to investigational use only and are not available for sale. For more information, please visit www.pqbypass.com