SALT LAKE CITY--(BUSINESS WIRE)--SINTX Technologies, Inc. (NASDAQ: SINT) (“SINTX” or the “Company”), an original equipment manufacturer of silicon nitride ceramic for medical and non-medical applications, announced new studies confirming that its material inactivates SARS-CoV-2, the causative agent of the COVID-19 pandemic, as well as the Influenza A virus that is responsible for the common cold.
Previous findings had shown that SINTX’s silicon nitride can inactivate SARS-CoV-2, as well as other single-strand RNA (ssRNA) viruses such as Influenza A, Feline calicivirus, and Enterovirus. Those findings were first reported in June 2020 on BioRxiv, a pre-print server for COVID-19 research ahead of peer review. Data related to the other ssRNA viruses are under peer review for publication.
The latest SARS-CoV-2 testing was done at the National Center for Biodefense and Infectious Diseases, George Mason University, Virginia (GMU). The study was designed to (1) reproduce the earlier findings using an independent institution and study protocol, and (2) show a time-and-dose-dependent antiviral effect of silicon nitride, and (3) establish a U.S.-based testing facility for future testing of commercial products, such as fabrics containing silicon nitride.
The GMU data confirm that silicon nitride strongly inhibits SARS-CoV-2, after just one minute of exposure. The anti-viral effect increases with higher doses and longer duration of exposure to silicon nitride. Importantly, silicon nitride is non-toxic to mammalian cells, a finding that is consistent with the successful clinical outcomes of SINTX’s spinal implants made of the same material as was tested at GMU.
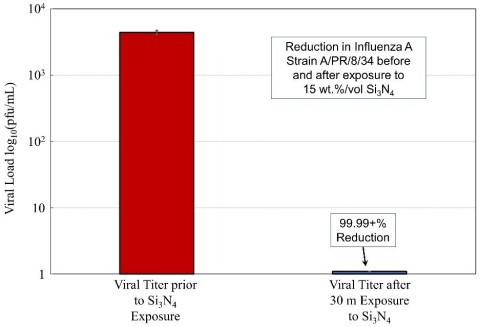
Separate testing at another contract research facility, ImQuest BioSciences, Frederick, Maryland, showed that SINTX’s silicon nitride strongly inactivates the Influenza A virus as well. Selected data from that testing is shown in the graph above.
“The ability of silicon nitride to effectively terminate the SARS-CoV-2 and Influenza A viruses is of importance, as the world braces for the flu season, with SARS-CoV-2 still around,” said Dr. Sonny Bal, President, and CEO of SINTX Technologies. “We are moving at full speed to incorporate silicon nitride into consumer masks that will catch-and-kill these respiratory pathogens. Beyond face masks, the antipathogenic advantages of silicon nitride will benefit schools, nursing homes, hospitals, casinos, cruise ships, day-care centers, commercial travel, and many other markets. Our R&D will advance the science, while external partners develop commercial products.”
“We are excited to get the results from this study as they solidify what we already know about SINTX and to continue efforts around bringing a mask to market,” said Bruce Lorange, CEO of O2TODAY, the company with whom SINTX has entered an agreement to develop face masks. “The latest data from SINTX is a solid confirmation of silicon nitride’s promise toward combatting the spread of COVID-19 and the flu, both of which are global worries.”
The COVID 19-driven demand for antipathogenic materials goes beyond face masks. As such, SINTX is identifying new revenue opportunities in industries such as automotive, aerospace, biomedicine, and others. The company is in discussions with prospective partners interested in developing a variety of products, such as automotive parts, cell phone cases, air filters, and medical PPE. SINTX looks forward to sharing additional developments in the coming months.
About SINTX Technologies, Inc.
SINTX Technologies is an OEM ceramics company that develops and commercializes silicon nitride for medical and non-medical applications. The core strength of SINTX Technologies is the manufacturing, research, and development of silicon nitride ceramics for external partners. The Company presently manufactures silicon nitride powders and components in its FDA registered and ISO 13485:2016 certified manufacturing facility.
For more information on SINTX Technologies or its silicon nitride material platform, please visit www.sintx.com.
About ImQuest BioSciences Inc.
ImQuest BioSciences, Inc. (http://imquestbio.com/), based in Frederick, Maryland, is a contract research organization (CRO) focused on assisting their biotechnology and pharmaceutical company clients in the development of agents for the prevention and treatment of infectious disease, cancer and inflammation. ImQuest’s proprietary ImQuestSUCCESS platform for virology, microbiology, topical microbicides, and cancer has assisted their clients in expediting the development of products for clinical evaluation through rapid identification of candidates with a high probability of clinical success by reducing the risk associated with failure experienced during clinical development.
Forward-Looking Statements
SINTX Technologies: This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) that are subject to a number of risks and uncertainties. Risks and uncertainties that may cause such differences include, among other things; the collaboration with O2TODAY may not result in the development of any products; that SINTX has not as yet developed any products with antiviral properties which incorporate the use of silicon nitride; products developed under the joint development agreement may not be effective against the SARS-CoV-2 virus; incorporation of silicon nitride into personal protective equipment may not be safe or effective; volatility in the price of SINTX’s common stock; the uncertainties inherent in new product development, including the cost and time required to commercialize such product(s); market acceptance of our products once commercialized; SINTX’s ability to raise additional funding and other competitive developments. Readers are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date on which they are made and reflect management’s current estimates, projections, expectations and beliefs. There can be no assurance that any of the anticipated results will occur on a timely basis or at all due to certain risks and uncertainties, a discussion of which can be found in SINTX’s Risk Factors disclosure in its Annual Report on Form 10-K, filed with the Securities and Exchange Commission (SEC) on March 26, 2020, and in SINTX’s other filings with the SEC. SINTX disclaims any obligation to update any forward-looking statements. SINTX undertakes no obligation to publicly revise or update the forward-looking statements to reflect events or circumstances that arise after the date of this report.