LAKE FOREST, Calif.--(BUSINESS WIRE)--STAAR Surgical Company (NASDAQ: STAA), a leading developer, manufacturer and marketer of implantable lenses and companion delivery systems for the eye, today announced that EVO Viva™, STAAR’s innovative presbyopia correcting implantable Collamer® lens (“ICL”), has been approved for sale. STAAR received CE Mark approval of the presbyopic indication for its EVO+ Visian® ICL with Aspheric (EDOF) Optic, commercially marketed as “EVO Viva”, from its European Notified Body, DEKRA, on July 2, 2020. EVO Viva will initially be available to patients through select eye doctors in Spain, Germany and Belgium. Broader availability of the lens will follow in Europe and other markets recognizing the CE Mark over the coming months. An introductory video highlighting EVO Viva’s market positioning for countries that accept the CE Mark is included with this press release.
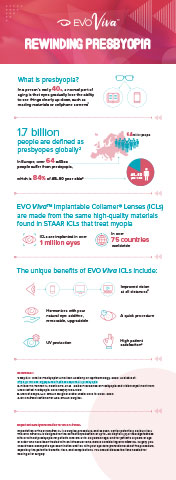
"The launch of EVO Viva significantly expands the market opportunity for our EVO family of lenses to now include lenses for patients suffering from presbyopia in the over 30 countries that currently recognize the CE Mark,” said Caren Mason, President and CEO of STAAR Surgical. “EVO Viva is a new treatment option for potential future consideration by the 1.7 billion people globally with presbyopia1 who may be burdened by reading glasses or frequent replacement contact lenses. EVO Viva reinforces our deep commitment to patients seeking visual freedom who have the express desire to Get Rid of their Reading Glasses. We believe EVO Viva can represent an attractive alternative among refractive surgery options currently available in the market today that require either ablating corneal tissue or removal of one’s healthy crystalline lens.2 EVO Viva further extends the renaissance of STAAR’s EVO family of lenses and encourages broader use of the entire EVO family of lenses.”
STAAR’s lenses have been improving patient vision for over 20 years and more than 1,000,000 ICLs have been implanted by surgeons with patient satisfaction reported at 99.4%.4 ICL’s are implanted within the posterior chamber, directly behind the iris, and in front of the anterior capsular bag. ICLs offer an intraocular alternative for the correction/reduction of refractive error in those who currently use spectacles and/or contact lenses for vision correction.
EVO Viva is STAAR's next-generation implantable Collamer lens. The lens is designed to work in harmony with a patient's eye for the correction or reduction of myopia and presbyopia in phakic and pseudo-phakic (post-cataract IOL) eyes to correct vision. The innovative EVO Viva lens adds near and intermediate vision correction for patients with presbyopia. Presbyopia is a normal age-related loss of vision that makes near objects difficult to see or blurry, which generally becomes noticeable beginning in the early-to-mid 40s and continues to worsen.3
A multicenter, prospective clinical investigation demonstrated the ability of the EVO Viva lens to correct myopia and presbyopia, resulting in improvement of uncorrected near, intermediate and distance visual acuity without compromising quality of vision. Subjects reported significant improvements in quality of life and high levels of spectacle independence and satisfaction. A clinical paper authored by the Medical Monitor and Principle Investigators from the study is being submitted for publication.
The Directions for Use (DFU) lists the indications as: EVO Viva TM ICL (Implantable Collamer ® Lens) with Aspheric (EDOF) Optic is indicated for use in phakic eye treatment in patients 21– 60 years of age and pseudophakic eye treatment in patients with monofocal IOLs with and without cylinder correction 21 years of age and older for:
- The correction/reduction of myopia in patients ranging from -0.5 D to -20.0 D at the spectacle plane.
-
The correction/reduction of myopia with presbyopia in patients ranging from -0.5 D to -20.0 D at the spectacle plane.
- For extended depth of focus and improved near visual acuity.
- With an anterior chamber depth (ACD) equal to or greater than 2.8 mm as measured from the corneal endothelium to the anterior lens capsule.
1 Fricke, Global Prevalence of Presbyopia and Vision Impairment from Uncorrected Presbyopia, 2018.
2 Laser vision correction procedures ablate corneal tissue and refractive-clear lens exchange procedures require the removal of one’s healthy crystalline lens.
3 Mayo Clinic, Patient Care & Health Information, Diseases & Conditions, Presbyopia, December 2017.
4 Data on file.
About STAAR Surgical
STAAR, which has been dedicated solely to ophthalmic surgery for over 30 years, designs, develops, manufactures and markets implantable lenses for the eye, with companion delivery systems. These lenses are intended to provide visual freedom for patients, lessening or eliminating the reliance on glasses or contact lenses. All of these lenses are foldable, which permits the surgeon to insert them through a small incision. STAAR’s lens used in refractive surgery is called an Implantable Collamer® Lens or “ICL,” which includes the EVO Visian ICL™ product line. More than 1,000,000 Visian® ICLs have been implanted to date and STAAR markets these lenses in over 75 countries. To learn more about the ICL go to: www.discovericl.com. Headquartered in Lake Forest, CA, the company operates manufacturing and packaging facilities in Aliso Viejo, CA, Monrovia, CA and Nidau, Switzerland. For more information, please visit the Company’s website at www.staar.com.
Safe Harbor
All statements in this press release that are not statements of historical fact are forward-looking statements, including statements about any of the following: the market opportunity for EVO Viva, the potential financial impact of EVO Viva’s availability, product safety or effectiveness, and any statements of assumptions underlying any of the foregoing. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include risks and uncertainties related to the COVID-19 pandemic and related public health measures, as well as the factors set forth in the Company’s Quarterly Report on Form 10-Q for the quarter ended April 3, 2020, and Annual Report on Form 10-K for the year ended January 3, 2020 under the caption “Risk Factors,” which is on file with the Securities and Exchange Commission and available in the “Investor Information” section of the company’s website under the heading “SEC Filings.” We disclaim any intention or obligation to update or revise any projections or forward-looking statement due to new information or events. These statements are based on expectations and assumptions as of the date of this press release and are subject to numerous risks and uncertainties, which could cause actual results to differ materially from those described in the forward-looking statements. The risks and uncertainties include, among others, the following: global economic conditions; the discretion of regulatory agencies to approve or reject existing, new or improved products, or to require additional actions; international trade disputes; and the willingness of surgeons and patients to adopt a new or improved product and procedure. The Visian ICL with CentraFLOW, known as EVO Visian ICL, and EVO Viva not currently approved for sale in the United States.
Important Safety Information for EVO Viva ICL
Implantation of the EVO Viva ICL is a surgical procedure, and as such, carries potentially serious risks. The EVO Viva ICL is designed for the correction/reduction of up to -20 diopters (D) of nearsightedness with or without presbyopia for patients who are 21 to 60 years of age; and for patients 21 years of age or older who have been treated with an intraocular lens. Before considering EVO Viva ICL surgery you should have a complete eye examination and talk with your eye care professional about the procedure, especially the potential benefits, risks, and complications. You should discuss the time needed for healing after surgery.