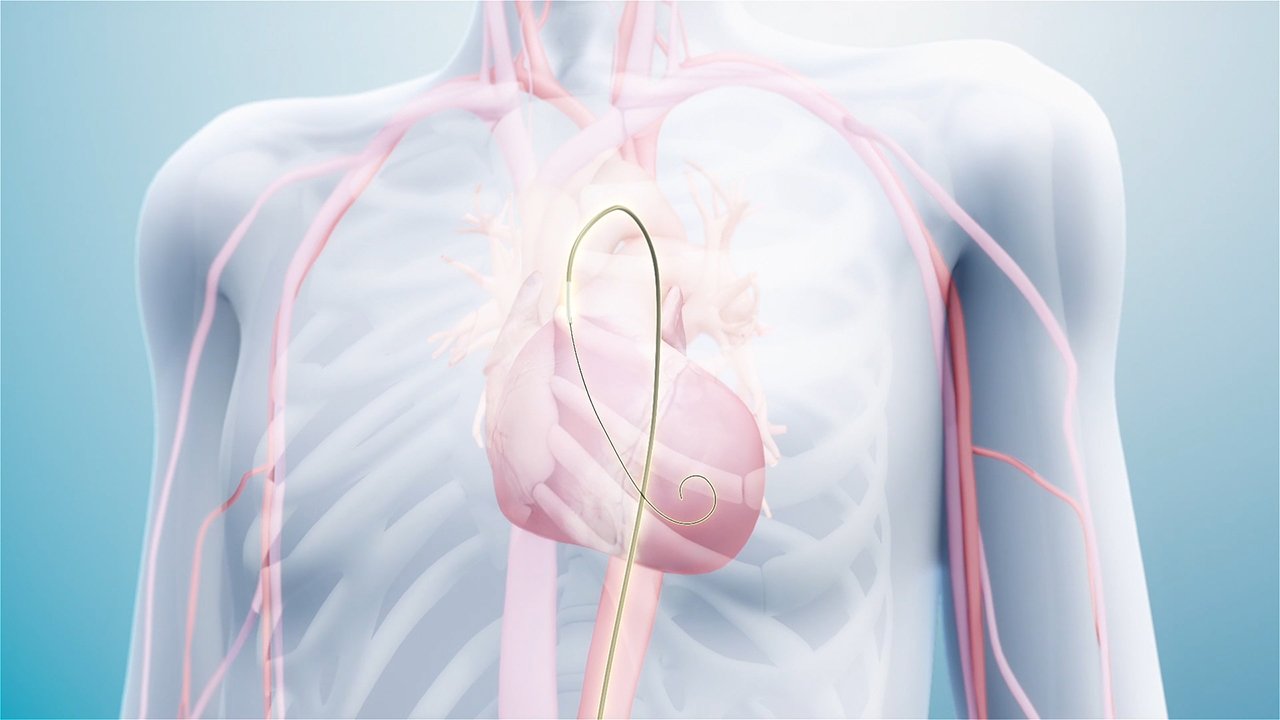
SANTA CLARA, Calif.--(BUSINESS WIRE)--Ancora Heart, Inc., a company developing a novel therapy to address heart failure, today announced U.S. Food and Drug Administration (FDA) approval of its Investigational Device Exemption (IDE) application for the CorCinch-HF pivotal study, which is designed to evaluate the safety and efficacy of the AccuCinch® Ventricular Restoration System in patients with heart failure and reduced ejection fraction (HFrEF). The AccuCinch System is the first completely percutaneous device designed to directly reshape the left ventricle of the heart, thereby addressing the fundamental issue in the progression of systolic heart failure.
“Patients with heart failure are in need of improved solutions and the AccuCinch technology is unique as it addresses heart function from the perspective of ventricular reshaping, with a minimally invasive approach, and potentially at an earlier phase before progression to advanced disease,” said Martin B. Leon, MD, professor of medicine and Director of the Center for Interventional Vascular Therapy at Columbia University Irving Medical Center and NewYork-Presbyterian, and chairman of the CorCinch-HF study. “The CorCinch clinical programs are developing a new class of therapy and the clinical trials have embraced a strong collaboration between Heart Failure and Structural Heart teams. We are encouraged by the clinical benefits demonstrated in the CorCinch feasibility phase and are looking forward to the CorCinch-HF randomized study to confirm these findings.”
“Heart failure is a chronic and progressive condition that often worsens past the clinical effectiveness of existing treatments, leaving patients with few options as their disease advances,” said Ulrich Jorde, MD, co-principal investigator of the CorCinch-HF pivotal study; professor of medicine, Albert Einstein College of Medicine, and section head of Heart Failure, Cardiac Transplantation and Mechanical Circulatory Support at Montefiore Health System in New York. “Early data on the AccuCinch procedure show that it may enable us to address ventricular function via a completely transcatheter approach, which we hope will translate into improved clinical outcomes and quality of life.”
The CorCinch-HF pivotal study is a prospective, randomized, open-label, multicenter, international, clinical safety and efficacy investigation of the AccuCinch System, which is designed to enroll 400 patients at up to 80 centers worldwide. The study has a unique design allowing initial analysis of safety and clinical efficacy for PMA submission after the first 250 patients have reached six months of follow-up, and then a second analysis after the entire cohort has reached twelve months of follow-up. The study will ultimately follow patients through five years post treatment to track long-term results.
“The prospect of addressing heart failure with a percutaneous approach provides patients an additional therapeutic option. The CorCinch early feasibility trials created foundational data that guided us to identify the patients to study in the randomized trial,” said Mark Reisman, MD, co-principal investigator of the CorCinch-HF pivotal study and section head of Interventional Cardiology at the UW Medicine Heart Institute in Seattle.
The transcatheter AccuCinch System is designed to complement and enhance the existing care cardiologists provide to manage symptoms and slow or stop the progression of heart failure. For patients where heart failure has progressed beyond the ability of medications and pacemakers to manage symptoms, minimally invasive percutaneous device therapy with the AccuCinch System may provide an effective treatment option.
“The approval of the IDE for the CorCinch-HF pivotal trial represents a major milestone as we continue to gather data to evaluate the safety and effectiveness of the AccuCinch System,” said Jeff Closs, President and CEO of Ancora Heart. “We look forward to working with study sites to initiate patient enrollment as soon as possible.”
About Heart Failure
An estimated 6.5 million U.S. adults live with heart failure, a condition in which the heart’s muscles slowly weaken and lose their ability to pump enough oxygen-rich blood to the body.1 Heart failure patients suffer from debilitating symptoms including persistent exhaustion, trouble breathing, confusion and loss of memory. About half of heart failure patients have an enlarged left ventricle, the main pumping chamber of the heart, which causes more stress on the heart and leads to reduced pumping efficiency. Current heart failure treatments only partially address the enlarged left ventricle and up to 50 percent of people who develop heart failure die within five years of diagnosis.1
About the AccuCinch Ventricular Restoration System
The AccuCinch Ventricular Restoration System is a fundamentally different and innovative therapy for patients with heart failure. During the AccuCinch procedure, an implant is placed into the mid left ventricular wall, the implant is then cinched. Once cinched, the implant is intended to reduce the size of the left ventricle, as well as support and strengthen the heart wall to help improve left ventricular function. The AccuCinch System is designed to provide extra stability and support to the left ventricular wall. This may lower stress on the heart and allow improved functional capacity and quality of life for patients.
About Ancora Heart
Ancora Heart, Inc., based in Santa Clara, Calif., is dedicated to helping people with heart failure feel better and live longer. Ancora Heart has developed the AccuCinch Ventricular Restoration System, an investigational device therapy designed to repair the enlarged left ventricle targeting the underlying cause of heart failure. The AccuCinch heart failure treatment was created to benefit the millions of patients who otherwise have no minimally invasive option available to them. For more information visit www.ancoraheart.com.
_______________________
1 Benjamin E.J., Blaha M.J., Chiuve S.E., et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135: pp. e146-e603
* Dr. Martin Leon owns equity in Ancora Heart.