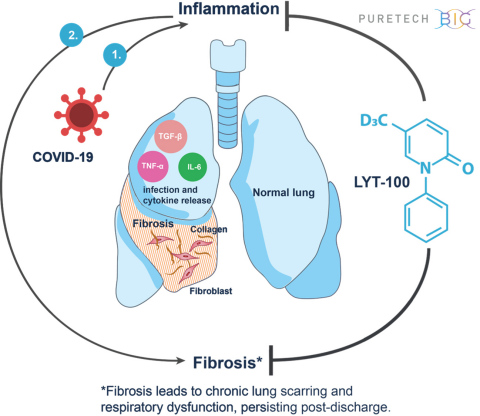
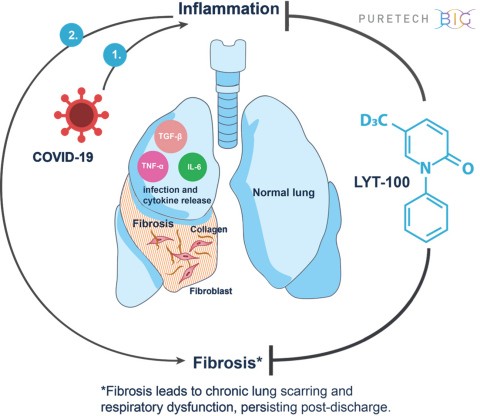
BOSTON--(BUSINESS WIRE)--PureTech Health plc (LSE: PRTC) (“PureTech” or the “Company”), a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, today announced plans to advance its wholly-owned clinical-stage product candidate LYT-100 (deupirfenidone) as a potential treatment for serious respiratory complications, including inflammation and fibrosis, that persist following the resolution of SARS-CoV-2 (COVID-19) infection. LYT-100 employs a multimodal mechanism of action to potentially reduce, delay or prevent the lung dysfunction that has recently been documented in COVID-19 patients, including those who have recovered from the infection. The global, randomized, placebo-controlled trial is expected to begin in Q3 2020 and will evaluate LYT-100 in non-critical COVID-19 patients with respiratory complications. Patients will continue treatment for up to three months.
“Hundreds of clinical trials are underway to combat COVID-19, but the vast majority are focused on vaccines or the acute treatment of severe patients,” said Dennis Ausiello, MD, former chief of medicine at Massachusetts General Hospital and a member of the PureTech R&D Committee. “As we learn more about the full impact of COVID-19 on the body, we’re seeing post-recovery, longer-term pulmonary dysfunction similar to that observed following infection with other coronaviruses, including SARS and MERS. In fact, emerging data suggest that a high proportion of COVID-19 patients are at risk of impaired lung function and fibrosis after recovery, as well as during acute infection. With more than five million documented infections to date worldwide, there is a clear and urgent need for therapeutics to address the longer-term sequalae of COVID-19.”
LYT-100 is an oral anti-fibrotic and anti-inflammatory small molecule. It is a deuterated analogue of pirfenidone. Oral pirfenidone is approved for the treatment of idiopathic pulmonary fibrosis (IPF) in the United States, European Union, Japan and a number of other countries and has received Breakthrough Therapy designation from the FDA for unclassifiable interstitial lung disease. In prior Phase 1, healthy volunteer studies, LYT-100 has shown a differentiated and superior pharmacokinetic profile compared to pirfenidone, suggesting improved efficacy, tolerability and safety, while retaining the same intrinsic pharmacology of pirfenidone. Preclinical research also shows that LYT-100 potently inhibits a range of pro-inflammatory cytokines including IL6, TNF alpha and TGF-beta.
“Many interstitial lung diseases (ILDs) are characterized by inflammation and fibrosis, which can result in impaired lung function and progressive pulmonary fibrosis. Interstitial pneumonia and ILD are manifestations of some viral infections in a subgroup of patients in the short term and may persist for months or years in another subgroup of patients who survive following severe acute respiratory syndrome (SARS) associated with coronavirus,” said Ganesh Raghu, MD, professor of medicine and adjunct professor of laboratory medicine at the University of Washington (UW) and director of the Center for Interstitial Lung Disease at UW Medicine. “Certain pathobiological features of COVID-19 are similar to features of interstitial pneumonia and acute exacerbations of fibrotic lung diseases. Therapeutic strategies targeting pathways of immune dysregulation, innate immunity, inflammation and fibrosis are appropriate to consider as potential treatments of COVID-19 and fibrotic ILD in the context of clinical trials.”
“PureTech has always been driven to help patients through innovation in medicine, and our LYT-100 program in COVID-19 represents another example of this commitment,” said Eric Elenko, PhD, chief innovation officer at PureTech. “The unique anti-fibrotic and anti-inflammatory properties of LYT-100 may have therapeutic potential in a range of conditions, including other interstitial lung diseases as well as lymphedema, for which a trial is planned to begin in 2020.”
PureTech expects to initiate a global, multi-center, randomized, double-blinded, placebo-controlled trial in Q3 2020 to evaluate the efficacy, safety and tolerability of LYT-100 in non-critical COVID-19 patients with respiratory complications. Patients will continue treatment for up to three months. The trial is expected to enroll approximately 150 patients, with a primary endpoint measuring pulmonary function testing. The trial will also assess exploratory endpoints including pharmacokinetics, acute inflammatory biomarkers, hospitalization events, imaging and patient-reported outcomes. PureTech expects to announce topline results in mid-2021.
As previously announced, in March 2020 PureTech initiated a Phase 1 trial of LYT-100 evaluating its safety, tolerability and the pharmacokinetic profile of multiple doses in healthy participants. Results from this trial are anticipated later this year, and a subsequent proof-of-concept trial in people with breast cancer-related, upper limb secondary lymphedema is expected to begin in 2020, with topline results expected in 2021. PureTech is also evaluating additional inflammatory and fibrotic conditions that could potentially be addressed with LYT-100.
About LYT-100
LYT-100 is PureTech’s most advanced wholly-owned product candidate. A deuterated form of pirfenidone, an approved anti-inflammatory and anti-fibrotic drug, LYT-100 is being developed for the potential treatment of a range of conditions involving fibrosis, inflammation and impaired lymphatic flow, including lymphedema, idiopathic pulmonary fibrosis (IPF), interstitial pneumonias, unclassifiable interstitial lung disease (uILD) and other interstitial lung disease (ILD), radiation-induced fibrosis and focal segmental glomerulosclerosis (FSGS). LYT-100 is currently being evaluated in a Phase 1 multiple ascending dose and food effect trial in healthy volunteers with a subsequent assessment in patients with breast cancer-related, upper limb secondary lymphedema expected to begin in 2020. PureTech also plans to initiate a trial in Q3 2020 evaluating LYT-100 as a potential treatment for serious respiratory complications, including inflammation and fibrosis, that persist following the resolution of SARS-CoV-2 (COVID-19).
About PureTech Health
PureTech is a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, including intractable cancers, lymphatic and gastrointestinal diseases, central nervous system disorders and inflammatory and immunological diseases, among others. The Company has created a broad and deep pipeline through the expertise of its experienced research and development team and its extensive network of scientists, clinicians and industry leaders. This pipeline, which is being advanced both internally and through PureTech’s Founded Entities, is comprised of 23 product candidates and one product that has been cleared by the US Food and Drug Administration (FDA). All of the underlying programs and platforms that resulted in this pipeline of product candidates were initially identified or discovered and then advanced by the PureTech team through key validation points based on the Company’s unique insights into the biology of the brain, immune and gut, or BIG, systems and the interface between those systems, referred to as the BIG Axis.
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Forward Looking Statement
This press release contains statements that are or may be forward-looking statements, including statements that relate to the company's future prospects, developments, and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks and uncertainties that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, those risks and uncertainties described in the risk factors included in the regulatory filings for PureTech Health plc. These forward-looking statements are based on assumptions regarding the present and future business strategies of the company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, neither the company nor any other party intends to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.