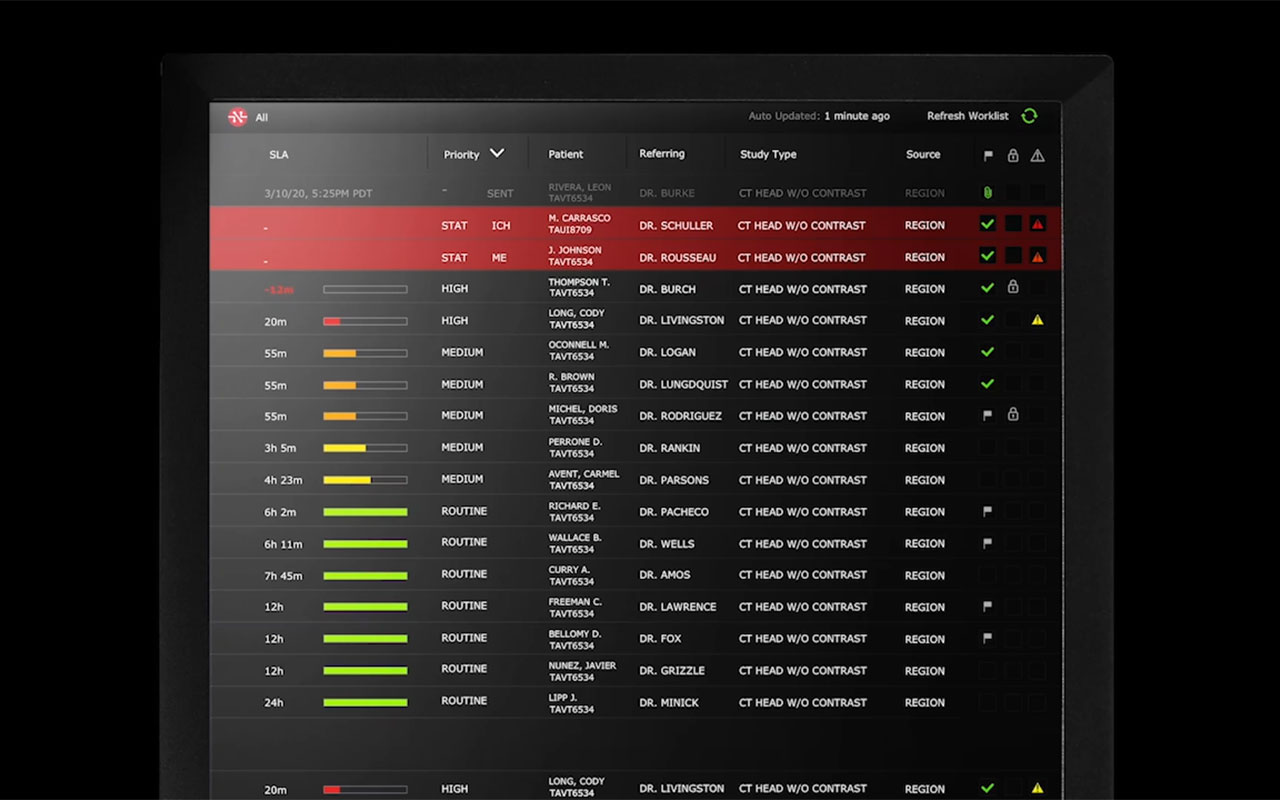
PALO ALTO, Calif.--(BUSINESS WIRE)--Nines today announced the NinesAI™ medical device making world-class artificial intelligence available to the rapidly-growing telehealth industry. NinesAI is FDA-cleared and supports the automated radiological review of CT Head images for the possible presence of two time-critical, life-threatening indications - intracranial hemorrhage and mass effect - to aid radiologists in triaging cases. Nines is the first company to receive U.S. Food and Drug Administration clearance for artificial intelligence technology that triages mass effect conditions, and to its knowledge is the first company to receive simultaneous FDA clearance on multiple indications. NinesAI will be deployed to radiologists in Nines’ teleradiology practice and will also be available to Nines’ customers for in-house use at no added cost.
Doctors and Engineers Ease Radiologists’ Burdens And Deliver Breakthrough AI
Radiologists suffer from high rates of burnout, according to MedScape’s Radiologist Lifestyle 2020 report, due to ever-increasing workload and suboptimal working conditions. Nines’ team includes world-class radiologists and top Silicon Valley engineers devoted to relieving those burdens via more efficient workflows that positively impact patient care. Together, they have developed the Nines Navigator™ worklist and the Nines Reading Assistant, which are administrative, non-medical device programs to improve radiologist focus. NinesAI assists radiologists by alerting them to the possible presence of intracranial hemorrhage and mass effect on head CT scans, life-threatening conditions that can be prioritized for review and consultation with hospital physicians treating patients.
“At Nines, we believe the application of advanced technology can address complex and pressing challenges in healthcare, and in particular for radiology, solve for a higher rate of burnout among radiologists,” said Nines co-founder and CEO, David Stavens. “With clearance from FDA, we’re proud to offer transformative AI innovation supporting the prioritization and triage of emergent conditions to complement radiologists’ work and ultimately improve the quality of patient care. We’re excited to partner with customers who seek cutting edge tools to deal with the conditions that matter most for patients.”
Nines Supports Its Teleradiology Customers with Free Access to NinesAI
The rise of the COVID-19 pandemic presents unparalleled circumstances and emerging challenges for patients and the healthcare community. Hospitals and healthcare providers are even more resource-constrained and continue to lack access to cutting edge technology that supports their critical work and care for patients.
All current customers and new customers who sign up with Nines before June 30, 2020 will get NinesAI for free. This access will allow Nines’ customers to assess, identify and triage emergent conditions of intracranial hemorrhage and mass effect while in-house radiology departments are inundated with diagnostics. For these emergent conditions, time is of the essence. With intracranial hemorrhage for example, the 30-day mortality rate ranges from 35% to 52% with only 20% of survivors expected to have full functional recovery at 6 months, and approximately half of this mortality occurs within the first 24 hours.* Time to intervention is critical, and radiologists using NinesAI can be notified of a potential life-threatening finding in approximately 15 seconds after the scan is complete and potentially life-saving care can be administered.
Nines’ radiologists have exceptional technology expertise, are well-respected medical professionals, and are the core of a growing community that enables technology to augment doctors’ - and, ultimately, patients’ - experiences in a truly pioneering fashion.
To learn more about Nines teleradiology practice and NinesAI, please visit https://www.nines.com.
About Nines
Nines, Inc. and affiliated professional entities do business under the Nines brand. Nines provides a better approach to teleradiology, improving patient care with an exceptional team of clinical experts, engineers, and data scientists. Nines is backed by Accel and 8VC and is based in Palo Alto, Calif. For more information, including career opportunities, visit https://www.nines.com.
*Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3443867/#R3