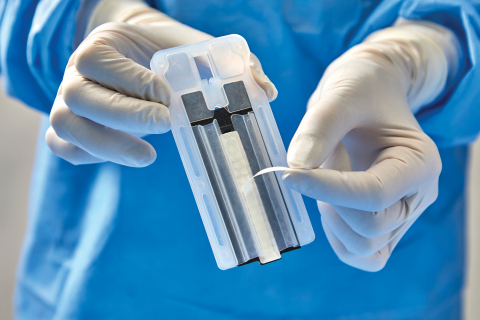
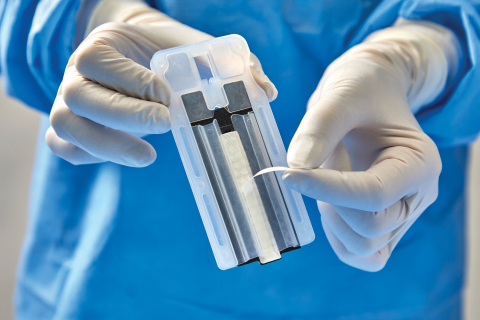
DEERFIELD, Ill.--(BUSINESS WIRE)--Baxter International Inc. (NYSE:BAX), a global leader in advancing surgical innovation, announced 510(k) clearance from the U.S. Food and Drug Administration for a new generation of its Peri-Strips Dry with Veritas Collagen Matrix (PSDV) product, known as PSDV with Secure Grip. Now available with “peel and secure” technology, PSDV is a staple line reinforcement material that bariatric surgeons have used to mitigate staple line complications for more than 15 years. This new generation of PSDV is two times faster to prepare compared to the previous version and another staple line reinforcement product.1
“We continue to advance innovation by providing surgeons tools that are faster and easier to use in the operating room,” said Wil Boren, president of Baxter’s Advanced Surgery business. “As surgical procedures are becoming more complex, it is important that surgeons have the tools they need to optimize patient care.”
Despite the sophistication of modern stapling technology used in bariatric procedures, staple line complications that result in high morbidity and mortality persist. The most prevalent complications are staple line bleeding and leaks. While the incidence of these complications is low, the impact can be clinically debilitating for the patient and costly to address.2
PSDV is proven to effectively reduce intraoperative and postoperative staple line bleeds and leaks in routine bariatric procedures such as a sleeve gastrectomy or gastric bypass. A 2015 meta-analysis extracted data from 295 studies predominantly comprised of morbidly obese patients undergoing laparoscopic gastric bypass and laparoscopic sleeve gastrectomy to include nearly 42,000 patients concerning bleeds and over 56,000 patients concerning leaks. PSDV showed statistically significant greater relative efficacy in reducing bleeding and leaks when compared to no staple line reinforcement, oversewing, and another staple line reinforcement product.2
PSDV with Secure Grip is applied to the surgical stapler via a pressure-sensitive adhesive strip. The new generation of PSDV provides strong adherence to the stapler, allowing surgeons to easily manipulate tissue. In a recent Staple Line Reinforcement Comparison study,1 30 bariatric nurses and surgical technicians evaluated stapler preparation time, ease of use and learnability of the PSDV with Secure Grip versus other options. Participants unanimously chose PSDV with Secure Grip as the most preferred staple line reinforcement material in the operating room.
PSDV with Secure Grip is available now to Baxter customers.
About Baxter
Every day, millions of patients and caregivers rely on Baxter’s leading portfolio of critical care, nutrition, renal, hospital and surgical products. For more than 85 years, we’ve been operating at the critical intersection where innovations that save and sustain lives meet the healthcare providers that make it happen. With products, technologies and therapies available in more than 100 countries, Baxter’s employees worldwide are now building upon the company’s rich heritage of medical breakthroughs to advance the next generation of transformative healthcare innovations. To learn more, visit www.baxter.com and follow us on Twitter, LinkedIn and Facebook.
Rx Only. For safe and proper use of this device, refer to the full Instructions for Use.
Important Safety Information
Peri-Strips Dry with Veritas Collagen Matrix with Secure Grip Technology (PSDV with Secure Grip) is intended for use as a prosthesis for the surgical repair of soft tissue deficiencies using surgical staplers when staple line reinforcement is needed.
PSDV with Secure Grip can be used for reinforcement of staple lines during gastric, bariatric, small bowel, colon and colorectal procedures.
Contraindications
The use of PSDV with Secure Grip is contraindicated in patients with known sensitivity to bovine or acrylic material.
This release includes forward-looking statements concerning Peri-Strips Dry with Veritas Collagen Matrix, including potential benefits associated with its use. The statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those in the forward-looking statements: satisfaction of regulatory and other requirements; actions of regulatory bodies and other governmental authorities; product quality, manufacturing or supply, or patient safety issues; changes in law and regulations; and other risks identified in Baxter's most recent filing on Form 10-K and other SEC filings, all of which are available on Baxter's website. Baxter does not undertake to update its forward-looking statements.
Baxter, Peri-Strips Dry and Veritas are registered trademarks of Baxter International Inc.
1 Baxter data on file. Staple Line Reinforcement Comparison Study with PSDV Secure Grip, PSDV Gel, and SEAMGUARD®
2 Shikora SA, Mahoney CB. Clinical Benefit of Gastric Staple Line Reinforcement (SLR) in Gastrointestinal Surgery: a Meta-analysis. Obes Surg. 2015 Jul;25(7):1133-41. This study was supported by a grant from Baxter.