CINCINNATI--(BUSINESS WIRE)--Aerpio Pharmaceuticals, Inc. (Nasdaq: ARPO), a biopharmaceutical company focused on developing compounds that activate Tie2 to treat ocular diseases and diabetic complications, today announced new results from the fifth cohort of subjects from a Phase 1b trial of a topical ocular formulation of AKB-9778 providing evidence of tolerability and IOP reduction from patients in that cohort with ocular hypertension (OHT) or primary open angle glaucoma (POAG).
The Phase 1b trial is a randomized, double-masked study designed to assess increasing concentrations of AKB-9778 dosed topically as eye drops. The primary outcome of the study is ocular safety and tolerability with change in intraocular pressure (IOP) at Day 7 as a pharmacodynamic outcome. Conjunctival hyperemia and IOP were assessed at 0 (pre-dose), 2, 4, and 8 hours post-dose on Day -1 (baseline), Day 1 (first day of AKB-9778 dosing) and Day 7 (last day of AKB-9778 dosing). Results from the first 3 cohorts, each comprising 12 subjects with normotensive eyes (non-glaucoma subjects), were reported on October 10, 2019. Subsequently, results from cohort 4, also containing 12 subjects with normotensive eyes, were observed to be consistent with those from cohorts 1 through 3.
Based on favorable tolerability and pharmacodynamic findings in these ocular normotensive subjects, Aerpio elected to recruit a fifth cohort of subjects with OHT/POAG on standard of care prostaglandin therapy to assess the safety, tolerability and pilot efficacy of once-daily AKB-9778 (40 mg/ml) as an adjunctive therapy. In the fifth cohort 43 patients were recruited with OHT/POAG and baseline IOP measurements between 17 and 27 mmHg while treated with once-daily prostaglandin therapy. Patients were randomized 3:1 to receive either AKB-9778 (32 subjects) or placebo (11 subjects), administered in the morning for 7 days, while continuing their evening prostaglandin therapy. Conjunctival hyperemia and IOP were assessed in the same manner as described for cohorts 1-4 (see above).
Results from cohort 5 are as follows:
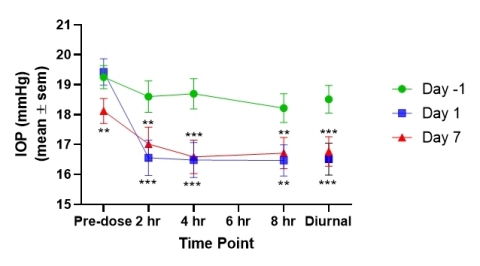
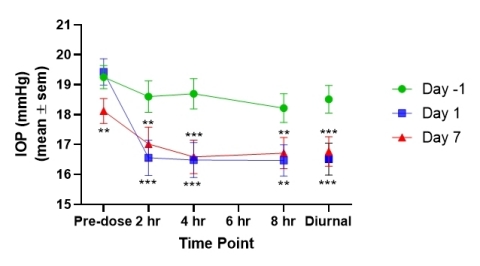
- Subjects in cohort 5 randomized to the active arm exhibited statistically significant decreases in IOP at all post-AKB-9778 administration time points on both Days 1 and 7 compared with Day -1 baseline values when they were being treated with prostaglandin alone (see Figure 1; Diurnal = mean IOP of 2, 4 and 8 hour time points; ** p < 0.05, *** p < 0.001).
- When the change is placebo-corrected, AKB-9778 plus prostaglandin versus prostaglandin alone produced a statistically significant decrease in IOP on Day 7 at 0, 4 and 8 hours post dose as compared to placebo (placebo-adjusted data at 0 hrs. -2.26 mmHg, p = 0.007; 2 hrs. -1.24, p = 0.138; 4 hrs. -1.47 mmHg; p = 0.048; 8 hrs. -1.80 mmHg; p = 0.041). We believe these results suggest a persistent IOP-lowering activity from adding AKB-9778 to standard-of-care prostaglandin therapy.
Topical ocular administration of AKB-9778 was well tolerated over seven days in cohort 5. In the active arm treated with AKB-9778 plus prostaglandin, 18.8% of subjects experienced hyperemia compared with 9.1% of subjects in the prostaglandin-alone arm. In all cases, this hyperemia was minimal-to-mild in severity, transient in duration and generally considered non-adverse. There were no reports of conjunctival hemorrhage or pain on instillation during the seven days of dosing.
“These early data suggest potential for a best in class profile for AKB-9778 to lower IOP when used in combination with prostaglandins,” said Brian Levy D.O., M.Sc., CEO of at Ocunexus Therapeutics and former CMO of Aerie Pharmaceuticals. “Although this is a small study, we observed IOP reductions in the fifth cohort of this trial that are comparable to or better than those produced by common commercial adjunctive therapies used in conjunction with prostaglandins. Moreover, AKB-9778 was well-tolerated through seven days of dosing with minimal hyperemia, no conjunctival hemorrhage and no discomfort on instillation. I look forward to the potential confirmation of these results in a larger and longer duration study.”
Pravin U. Dugel M.D., Partner, Retinal Consultants of Arizona, Clinical Professor, Roski Eye Institute, USC Keck School of Medicine, and Member of Aerpio’s Board of Directors said, “The opportunity to treat glaucoma via a novel mechanism-of-action that targets the Schlemm’s canal and the primary outflow channel may provide great benefit for patients beyond current therapies and definitely warrants further study. In addition to an impressive IOP-lowering activity on top of a prostaglandin, the encouraging tolerability profile observed from this cohort is also important for patient acceptance and compliance, as most current therapies have tolerability issues.”
Based on these results, Aerpio plans to further advance its glaucoma program through strategic alternatives such as a partnership or collaboration.
About AKB-9778
AKB-9778 binds to and inhibits vascular endothelial protein tyrosine phosphatase (VE-PTP), an important negative regulator of Tie2. Decreased Tie2 activity contributes to vascular instability in many diseases including diabetes. AKB-9778 activates the Tie2 receptor irrespective of extracellular levels of its binding ligands, angiopoietin-1 (agonist) or angiopoietin-2 (antagonist) and may be the most efficient pharmacologic approach to maintain normal Tie2 activation.
About Aerpio Pharmaceuticals
Aerpio Pharmaceuticals, Inc. is a biopharmaceutical company focused on advancing first-in-class compounds that activate Tie2 to treat ocular diseases and complications of diabetes. Tie2 is an important regulator of vascular stability, and its down-regulation, through activation of two inhibitors VE-PTP and Ang-2, is found in patients with diabetes and other conditions. The Company’s lead compound, AKB-9778, a first-in-class small molecule inhibitor of VE-PTP, is being investigated in an ongoing Phase 1b clinical trial as a topical drop formulation for its therapeutic potential in open-angle glaucoma. In the recently completed Phase 2b study (TIME-2b) AKB-9778 demonstrated the ability to lower proteinuria (as measured by decreasing urinary albumin creatine ratio, UACR) by about 20% replicating a finding in the previous Phase 2 study. The decrease in proteinuria suggests that AKB-9778 and our other Tie2 activating drug, ARP-1536, may have the potential to improve kidney function in diabetics potentially delaying progression to kidney dialysis. The Company’s second asset, ARP-1536 is a humanized monoclonal antibody observed to activate Tie2 receptors in a dose-dependent manner in preclinical models. Aerpio believes ARP-1536 holds potential as a monthly or biweekly systemic therapy to treat diabetic complications, including diabetic nephropathy. The Company’s third asset is a bispecific antibody that binds both VEGF and VE-PTP which inhibits VEGF activation and activates Tie2. This bispecific antibody has the potential to be an improved product for treating wet AMD and diabetic macular edema via intravitreal injection. Finally, the Company has exclusively out-licensed its fourth asset AKB-4924 (now called GB004), a first-in-class small molecule inhibitor of hypoxia-inducible factor-1 (HIF). GB004 is being developed by AKB-4924’s exclusive licensor, Gossamer Bio, Inc. (Nasdaq: GOSS), in return for an upfront payment of $20 million, future potential development, regulatory, and sales milestones of up to $400 million, and royalties on worldwide net sales. For more information, please visit www.aerpio.com.
Forward Looking Statements
This press release contains forward-looking statements. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, the Company’s product candidates, including AKB-9778, and the clinical development and therapeutic potential thereof, its plans to further the development of its glaucoma program, and the intended benefits from its collaboration with Gossamer Bio, Inc. for GB004. Actual results could differ from those projected in any forward-looking statements due to several risk factors. Such factors include, among others, potential disruptions in our business as a result of our exploration, review and pursuit of strategic alternatives or the public announcement thereof and any decision or transaction resulting from such review; the ability to continue to develop AKB-9778 or other product candidates; the inherent uncertainties associated with the drug development process, including uncertainties in regulatory interactions, commencing clinical trials and enrollment of patients in clinical trials; and competition in the industry in which the Company operates and overall market conditions; and the additional factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2018, as updated by our subsequent filings with the SEC. These forward-looking statements are made as of the date of this press release, and the Company assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law. Investors should consult all the information set forth herein and should also refer to the risk factor disclosure set forth in the reports and other documents the Company files with the SEC available at www.sec.gov.