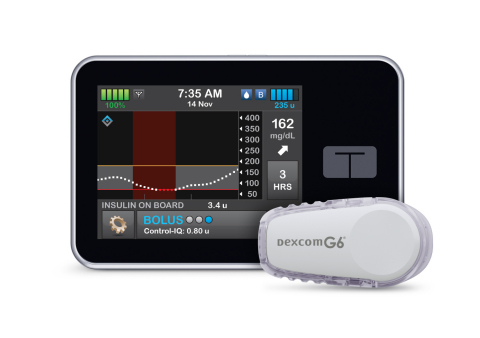
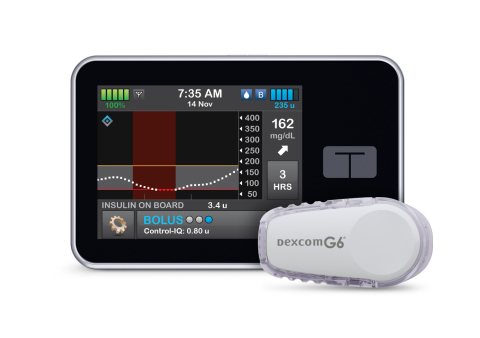
SAN DIEGO--(BUSINESS WIRE)--Tandem Diabetes Care, Inc. (NASDAQ: TNDM), a leading insulin delivery and diabetes technology company, today announced U.S. Food and Drug Administration (FDA) clearance of the t:slim X2™ insulin pump with Control-IQ™ technology, an advanced hybrid-closed loop feature designed to help increase time in range (70-180 mg/dL)1, and the first system cleared to deliver automatic correction boluses in addition to adjusting insulin to help prevent high and low blood sugar. The system integrates with Dexcom G6 continuous glucose monitoring (CGM), which requires no fingersticks for calibration or diabetes treatment decisions.2,3,4 Control-IQ technology for the t:slim X2 insulin pump is the first automated insulin dosing software in a new interoperable automated glycemic controller category that automatically adjusts insulin delivery to a person with diabetes by connecting to an alternate controller-enabled insulin pump (ACE pump) and integrated continuous glucose monitor (iCGM). This is the third category classified by the FDA for the interoperability of devices as a complete automated insulin dosing (AID) system.
All in-warranty t:slim X2 pump users in the United States will have the option to add the new feature free of charge via remote software update.5 The update is expected to be available by the end of January 2020, and new pumps with Control-IQ technology will begin shipping to customers in the same timeframe. The Company will continue to offer the t:slim X2 pump with Basal-IQ® predictive low glucose suspend technology as an option for people who prefer a system designed specifically to help prevent lows.
The t:slim X2 insulin pump with Control-IQ technology uses CGM values, in conjunction with other variables such as insulin on board, to predict glucose levels 30 minutes ahead and adjust insulin delivery accordingly. If glucose values are predicted to drop below 112.5 mg/dL, basal insulin delivery is reduced, and when predicted to be below 70 mg/dL, basal insulin delivery is stopped. If glucose values are predicted to be above 160 mg/dL in the next 30 minutes, basal insulin will be increased. If glucose values are predicted to be above 180 mg/dL, Control-IQ technology calculates a correction bolus with a target of 110 mg/dL, and delivers 60 percent of that value up to once an hour as needed. Control-IQ technology also offers optional settings for sleep and exercise that will change the treatment values to better match the different physiologic needs during these activities.
With the interoperable automated glycemic controller designation, the FDA establishes a new device class which includes special controls outlining requirements for this and future submissions in this category, as well as describes the reliability, device interoperability, cybersecurity, and clinical relevance data required to demonstrate acceptable performance. The t:slim X2 insulin pump was also the first to receive an ACE infusion pump classification in February 2019, and the first insulin pump designated as compatible with iCGM devices in June 2018.
More information on the new interoperable automated glycemic controller classification can be found in the press release issued by the FDA earlier today. https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-interoperable-automated-insulin-dosing-controller-designed-allow-more-choices
“Not only do new closed-loop systems need to be effective at improving glycemic control, they must also be easy to understand and use so patients can experience the full benefits of the technology. The t:slim X2 insulin pump with Control-IQ technology successfully achieved both objectives in the clinical studies,” said Boris Kovatchev, PhD, Director of the Center for Diabetes Technology at the University of Virginia and principal investigator of the International Diabetes Closed Loop (iDCL) trials. “We are very proud of the extensive academic research that went into this effort, and we are thrilled to see this work has translated into an FDA-cleared device for clinical use so that more people can experience the benefits of this technology.”
“With this clearance, we will be launching the most advanced automated insulin dosing system commercially available in the world today,” said John Sheridan, president and CEO of Tandem Diabetes Care. “This is a testament to our commitment to improving the lives of people with diabetes by offering simple-to-use products that deliver superior performance.”
“The approval of the Control-IQ system with Dexcom G6 CGM brings together two incredible products to deliver a powerful automated insulin dosing solution for people with diabetes,” said Kevin Sayer, president and CEO of Dexcom. “Sensor accuracy is a critical component for automated insulin dosing, and we are excited that Dexcom G6 users can benefit from our collaborative effort to integrate this advanced hybrid closed loop system.”
Benefits of Control-IQ Advanced Hybrid Closed-Loop Technology:
Predicts and helps prevent lows and highs – Control-IQ technology uses CGM readings to predict glucose values 30 minutes ahead and can increase, decrease or stop basal insulin delivery to help keep glucose in range (70-180 mg/dL).1
Automatic Correction Boluses – If glucose values are predicted to be above 180 mg/dL, Control-IQ technology calculates a correction bolus with a target of 110 mg/dL and delivers 60 percent of that value. It will do this up to once an hour as needed.
Accommodates for sleep and exercise – Control-IQ technology offers optional settings for sleep and exercise that change the treatment values to better match the different physiologic needs during these activities.
No fingersticks – With Dexcom G6 CGM integration, the Control-IQ feature works with no fingersticks required for mealtime dosing or calibration.2 Other benefits of the Dexcom G6 CGM include an extended 10-day wear, acetaminophen blocking6, and the ability to share real-time CGM data with up to 10 followers.7
Easy to use – The system has no complicated criteria to keep Control-IQ technology on. If the CGM signal is temporarily lost, the Control-IQ feature will resume automatically when the CGM is back in range. In the pivotal study, participants gave Control-IQ technology a 4.7 out of 5.0 for ease of use, and a 4.8 out of 5.0 for desire to continue use of the system.8
Standard Features of the t:slim X2 Insulin Pump:
Color touchscreen – The large color touchscreen on the t:slim X2 pump is easy to read, simple to learn, and intuitive to use for anyone familiar with a smartphone or tablet.
Small and discreet – The t:slim X2 pump is up to 38 percent smaller than other pumps9, yet can hold up to 300-units of insulin.
Can be used with or without the Control-IQ feature or CGM – When advanced features are turned off, the t:slim X2 pump removes the CGM chart from the screen and puts the Bolus and Option buttons front and center for easy access.
#1-rated customer support – Tandem Diabetes Care customer support has been consistently ranked number one by pump users in independent patient surveys since 2013.10
For additional product and safety information, or to begin the order process, visit http://tandemdiabetes.com/controliq
Free Demo App – Control-IQ Technology
Tandem’s free t:simulator™ App lets people experience the touchscreen interface of the t:slim X2 pump with Control-IQ technology directly on a mobile device. For more information and to download the app, visit http://www.tandemdiabetes.com/tsimulator.
Free Software Update for Current t:slim X2 Pump Users
All in-warranty t:slim X2 pump users in the United States will have the option to update their pumps with the Control-IQ feature free of charge via a software update using a personal computer. The Control-IQ feature update will require a user to secure a new prescription from his or her healthcare provider and complete an online training module. Internet and computer access are required for pump updates. All eligible t:slim X2 pump users will receive an email with more information on the update process in the coming days. Tandem expects the Control-IQ software update to be available for current t:slim X2 pump by the end of January 2020. Information about the requirements and update process is available at www.tandemdiabetes.com/X2update.
Control-IQ Technology – Clinical Outcomes
Data from the NIH-funded DCLP3 study, published by the New England Journal of Medicine in October 2019, compared use of a t:slim X2 insulin pump with Control-IQ technology and Dexcom G6 CGM integration (n=112) to a control group using a t:slim X2 pump with just Dexcom G6 CGM integration (n=56).8 This study was the first large-scale 6-month closed loop study to include a dedicated control group. There were no exclusion criteria based on hemoglobin A1c, history of acute complications, or previous experience using an insulin pump, and all participants completed the study. Those using Control-IQ technology experienced 71 percent time in range (70-180 mg/dL) per day on average compared to 59 percent in the control group.1 Sensor time spent above 180 mg/dL was 27 percent per day on average with Control-IQ technology and 39 percent in the control group. Sensor time spent below 70 mg/dL was 1.4 percent per day on average with Control-IQ technology and 1.9 percent in the control group. The system remained connected to CGM with Control-IQ on and active an average of 92 percent of the 26-week study period. In a five-point survey at the end of the study, users overwhelmingly rated the Control-IQ system as simple to use, giving it a 4.5 for trust, a 4.7 for ease of use, and a 4.8 for desire to continue using the system.
Important Safety Information for the t:slim X2 Insulin Pump with Control-IQ Technology
Caution: Federal (USA) law restricts the t:slim X2 insulin pump and Control-IQ technology to sale by or on the order of a physician.
Indications for Use:
t:slim X2 insulin pump
The t:slim X2 insulin pump with interoperable technology is an alternate controller enabled (ACE) pump that is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is indicated for use in individuals 6 years of age and greater. The pump is intended for single patient, home use and requires a prescription. The pump is indicated for use with NovoLog or Humalog U-100 insulin.
Control-IQ technology
Control-IQ technology is intended for use with a compatible integrated continuous glucose monitor (iCGM, sold separately) and ACE pump to automatically increase, decrease, and suspend delivery of basal insulin based on CGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ technology is intended for the management of Type 1 diabetes mellitus in persons 14 years of age and greater. Control-IQ technology is intended for single patient use. Control-IQ technology is indicated for use with NovoLog or Humalog U-100 insulin.
Contraindications and Warnings:
BOXED WARNING: Control-IQ technology should not be used by anyone under the age of six years old. It should also not be used in patients who require less than 10 units of insulin per day or who weigh less than 55 pounds.
Control-IQ technology is not indicated for use in pregnant women, persons on dialysis, or critically ill patients.
Users of the t:slim X2 pump and Control-IQ technology must:
- be able and willing to use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use;
- test blood glucose levels as recommended by their healthcare provider;
- demonstrate adequate carb-counting skills;
- maintain sufficient diabetes self-care skills;
- see healthcare provider(s) regularly; and
- have adequate vision and/or hearing to recognize all functions of the pump, including alerts, alarms, and reminders;
The t:slim X2 pump, transmitter, and sensor must be removed before MRI, CT, or diathermy treatment. For additional important safety information, visit tandemdiabetes.com/safetyinfo.
Insulin Pump Use and Diabetes
Diabetes is a chronic, life-threatening disease that affects more than 29 million people in the United States, or nearly 1 in 10 Americans. Tandem estimates that more than three million people in the United States require daily administration of insulin and are candidates for pump therapy. More than 425,000 Americans with type 1 diabetes use an insulin pump, or approximately 27 percent of the type 1 diabetes population. In addition, approximately 125,000 Americans with type 2 diabetes use an insulin pump, a small fraction of the type 2 diabetes population.
About Tandem Diabetes Care, Inc.
Tandem Diabetes Care, Inc. (www.tandemdiabetes.com) is a medical device company dedicated to improving the lives of people with diabetes through relentless innovation and revolutionary customer experience. The Company takes an innovative, user-centric approach to the design, development and commercialization of products for people with diabetes who use insulin. Tandem manufactures and sells the t:slim X2 insulin pump with Control-IQ technology. The t:slim X2 pump is capable of remote feature updates using a personal computer, and is the only automated insulin dosing device approved for children as young as 6 years old. Tandem is based in San Diego, California.
Tandem Diabetes Care and Basal-IQ are registered trademarks, and t:slim X2, Control-IQ and t:simulator are trademarks of Tandem Diabetes Care, Inc. Dexcom and Dexcom G6 are registered trademarks of Dexcom, Inc. All other third-party marks are the property of their respective owners.
Forward Looking Statement
This press release contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that concern matters that involve risks and uncertainties that could cause actual results to differ materially from those anticipated or projected in the forward-looking statements. These forward-looking statements relate to, among other things, the anticipated timing for the commercial launch of the t:slim X2 pump with Control-IQ technology and our ability to offer the Control-IQ software update for current t:slim X2 pump users. These statements are subject to numerous risks and uncertainties, including our ability to commence commercial scale manufacturing of the t:slim X2 pump with Control-IQ technology, our ability to operate and maintain a system to facilitate online training and prescription handling for existing t:slim X2 pump customers upgrading their existing devices, and the risk that we may encounter other challenges that may delay the commercial launch of t:slim X2 pump with Control-IQ technology, as well as other risks identified in our most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, and other documents that we file with the Securities and Exchange Commission. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this release. Tandem undertakes no obligation to update or review any forward-looking statement in this press release because of new information, future events or other factors.
_______________________
1 As measured by CGM
2 If glucose alerts and CGM readings do not match symptoms or expectations or if taking over the recommended maximum dosage amount of 1000mg of acetaminophen every 6 hours, use a blood glucose meter to make diabetes treatment decisions.
3 Dexcom G6 CGM sold separately
4 The Dexcom G6 CGM transmitter can only be paired with one medical device (either a Dexcom receiver or t:slim X2 pump) and one consumer device (phone or tablet) at the same time.
5 A new prescription and additional training are required for this software update.
6 Dexcom G6 CGM readings can be used to make diabetes treatment decisions when taking up to a maximum acetaminophen dose of 1,000mg every 6 hours. Taking a higher dose may affect the G6 readings.
7 Separate Follow App required.
8 Brown SA, Kovatchev D, Raghinaru JW, et al. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N Engl J Med. 2019;381(18):1707-17. DOI: 10.1056/NEJMoa1907863
9 38 percent smaller than MiniMed 630G and 670G and at least 28 percent smaller than MiniMed 530G, Animas Vibe and Omnipod System. Data on file, Tandem Diabetes Care.
10 dQ&A USA Diabetes Connections Surveys, 2013-2019