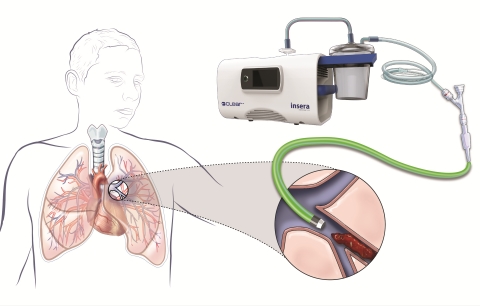
SACRAMENTO, Calif.--(BUSINESS WIRE)--Insera Therapeutics, Inc. announced today that the first patient with acute pulmonary embolism, namely life-threatening blood clots blocking lung arteries, has been treated with its flagship cyclical aspiration system, CLEAR. Earlier last month, the first two stroke patients were treated using the same platform. The company received Conformité Européenne (CE) Mark approval for its flagship product, the CLEAR Aspiration System, in March of 2019.
“Due to the large clot burden in the lung arteries, the 65-year old gentleman presented with right-sided heart failure and was in critical condition. Traditional treatments using blood thinning medications such as anti-coagulation and thrombolytic medications were contraindicated in this patient. We successfully treated this patient with massive bilateral acute pulmonary embolism using Insera’s CLEAR Aspiration System,” said Luka Novosel, MD PhD, Interventional Radiologist at the Sisters of Charity Hospital, Zagreb, Croatia. “I am very pleased with the ease of use of cyclical aspiration and its ability to rapidly ingest large amounts of clot. It also provides a safer mechanical means of removing life-threatening blood clots without the bleeding risk associated with blood thinning medications.”
Insera’s first-in-human success in treating pulmonary embolism comes on the heels of the recent announcement of the EXTRACT-PE trial results presented at the Vascular InterVentional Advances (VIVA) meeting held November 4-7, 2019 in Las Vegas, Nevada. This trial advances treatment options for the industry as it establishes the use of vacuum aspiration in patients with acute pulmonary embolism.
The CLEAR Aspiration System has European regulatory approval for the aspiration of blood clots, and is indicated for use for acute ischemic stroke secondary to large vessel occlusive disease, as well as the aspiration of blood clots from arteries (e.g. peripheral artery disease, pulmonary embolism, and coronary artery disease), and for the aspiration of blood clots from veins (e.g. in patients with deep venous thrombosis, cerebral venous sinus thrombosis or patients with renal failure who have clotted hemodialysis grafts).
Venous thromboembolism (VTE) refers to a blood clot that starts in a vein. It is the third leading vascular diagnosis after heart attack and stroke. A Deep Vein Thrombosis (DVT) is a blood clot that develops in one or more of the deep veins in the body, such as the legs, thighs, or arms. When a DVT clot breaks free from a vein wall, and travels to the lungs and blocks some or all of the blood supply, it is called a Pulmonary Embolism (PE). In patients with a pre-existing hole in the heart, the DVT clots that break free can travel to the brain (paradoxical embolism), and cause strokes.
“To date, we have successfully used the CLEAR Aspiration System in nine stroke patients. I am very pleased with the higher trend of first-pass recanalization using cyclical aspiration. For example, in our very first patient, we had a 6 minute procedure time from groin to recanalization with TICI 3 on first-pass (complete vessel re-opening on the first operative attempt),” said Vladimir Kalousek, MD, EBNI accredited Interventional Neuroradiologist at the Sisters of Charity Hospital, Zagreb, Croatia who has pioneered the use of cyclical aspiration.
“There is a huge unmet need for innovative devices for the removal of life-threatening blood clots. Our unique and proprietary cyclical aspiration technology has been shown to improve first-pass recanalization,1 reduce distal embolization,1 and has an improved safety profile at the time of the procedure,2” said Vikram Janardhan, Chief Executive Officer of Insera Therapeutics, Inc. “In the United States alone, it is estimated that every year there are around 225K patients with ischemic strokes due to large vessel occlusions (LVOs), 300K patients with PE, 600K patients with DVTs, and 100K patients with acute limb ischemia.”
About Insera - Insera Therapeutics, Inc. is an ISO 13485 certified medical device company focused on treatments for vascular diseases including stroke. CLEARTM stands for Cyclical, Luminal, Evacuation, Aspiration, and Retrieval. The CLEARTM Aspiration System is currently not cleared to market in the United States. InseraTM, CLEARTM, and CLEARTM Aspiration System are trademarks of Insera Therapeutics, Inc.
1. Arslanian RA, Marosfoi M, Caroff J, King RM, Raskett C, Puri AS, Gounis MJ, Chueh J. “Complete clot ingestion with cyclical ADAPT increases first-pass recanalization and reduces distal embolization.” J Neurointerventional Surg. 2019;0:1-6.
2. Kadirvel R, Dai D, Brinjikji W, Kallmes DF. “Cyclical aspiration has an improved safety profile compared to continuous uniform aspiration: In-vivo Randomized Study.” Presented at the 12th Annual Meeting of the Society of Vascular & Interventional Neurology (SVIN) held November 20-23, 2019 in Atlanta, Georgia.