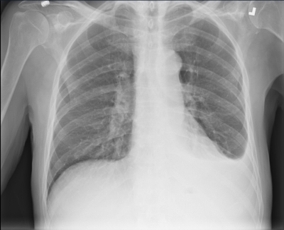
KIBBUTZ SHEFAYIM, Israel--(BUSINESS WIRE)--Zebra Medical Vision, the deep-learning medical imaging analytics company, announces today its fourth FDA 510(k) clearance for the HealthCXR device intended for the identification and triaging of pleural effusion in Chest X-rays. The recently cleared AI product expands its growing AI1™ bundle of triage and prioritization applications for Chest X-rays. The company will be showcasing its AI1™ bundle at this year's RSNA conference. Zebra-Med’s new solution automatically identifies findings suggestive of pleural effusion based on CR, DR, and/or DX scans, and notifies radiologists, enabling them to better address and prioritize cases.
Pleural effusion is an abnormal accumulation of fluid within the pleura and is referred to as “water in the lungs.” The condition can be caused by a variety of medical issues, including several acute conditions, such as severe pneumonia, trauma, and pulmonary edema. By providing the radiologist with additional insights of pleural effusion, Zebra-Med’s newest solution can facilitate a quicker diagnosis for the patients more likely to have acute pathology, resulting in more efficient treatment, and optimized patient care.
"Based on the real-world application of this product, we saw that Zebra-Med's automatic identification of pleural effusion on Chest X-rays can play a significant role in triage. It could be a relevant indicator for acute cardiopulmonary disease, so that clinical management can be adopted, as soon as possible, in order to provide optimal patient care," says Dr. J.J. Visser, Radiologist and Head of Imaging IT and Value-Based Imaging at Erasmus MC University Medical Center.
Zebra-Med is now part of the Digital Health Software Precertification (Pre-Cert) Pilot Program. As outlined by the FDA, the program will help inform the development of a future regulatory model that will provide a more streamlined and efficient oversight of software-based medical devices. In the Pre-Cert program, the FDA is proposing that software products from pre-certified companies will continue to meet the same safety and efficacy standards that the agency expects for products that have followed the standard path to market.
“We are happy with the 4th FDA nod for an additional medical solution that will leverage AI in healthcare, and improve patient care,” says Eyal Gura, Co-Founder and CEO of Zebra Medical Vision.“Adding a greater number of capabilities to our Chest X-ray package is key for increasing doctors’ trust, and the use of AI.”
Read more on Zebra-Med’s blog: https://zebramedblog.wordpress.com/no-smoke-without-fire
Zebra Medical Vision will be showcasing its AI1™ bundle, 2020 version, at RSNA 2019 [booth number 10527]. More information on the location of the booth can be found here.
About Zebra Medical Vision
Zebra Medical Vision’s imaging analytics platform allows healthcare institutions to identify patients at risk of disease and offer improved, preventative treatment pathways, to improve patient care. The company is funded by Khosla Ventures, Marc Benioff, Intermountain Investment Fund, OurCrowd Qure, Aurum, aMoon, Nvidia, J&J and Dolby Ventures. Zebra Medical Vision has raised $52 million in funding to date, and was named a Fast Company Top-5 AI and Machine Learning company. Zebra-Med leads the way in AI FDA cleared products, and is installed in hospitals globally, from Australia to India, Europe to the U.S, and the LATAM region.