REDWOOD CITY, Calif.--(BUSINESS WIRE)--Pulmonx Corporation, a leader in therapeutic pulmonary device technologies, announced today that the 2020 update from the Global Initiative for Chronic Obstructive Disease (GOLD) has upgraded the evidence level rating for bronchoscopic lung volume reduction (BLVR) with endobronchial valves, including the Zephyr Valve, for the treatment of emphysema / chronic obstructive pulmonary disease (COPD). In the newly released report, GOLD upgraded the evidence rating to “A”, the highest evidence rating, for BLVR with endobronchial valves.1 This evidence rating is based on results from well-designed randomized clinical trials with data from more than two clinical trials involving a substantial number of patients, including those treated with the Zephyr Valve. The Zephyr Valve is a minimally-invasive treatment option that has been shown to improve quality of life of emphysema patients by allowing them to experience less shortness of breath and be more active.2
GOLD’s Global Strategy for Diagnosis, Management and Prevention of COPD report is reviewed and revised annually by leading physicians in the field of COPD around the globe. It is used worldwide by healthcare professionals as a “strategy document” and tool in the management and prevention of COPD, a disease that impacts more than 65 million people globally. GOLD first added mention of the benefits of broncoscopic interventions for COPD, including endobronchial valves like the Zephyr Valve, in 2017. The 2020 report upgrades endobronchial valves to the best evidence level available, making it the GOLD standard of care for qualifying patients. The upgraded evidence rating of “A” for endobronchial valves signals that:
- Endobronchial valve treatment for emphysema is supported by a rich body of high-quality evidence, and is now a compelling alternative to lung volume reduction surgery (also evidence level A);
- Endobronchial valves, like the Zephyr Valve, are now distinguished from other bronchoscopic interventions mentioned in the report (such as coils, vapor) because they are the only bronchoscopic intervention to receive a GOLD evidence rating of “A”.
- In select patients with advanced emphysema, bronchoscopic interventions reduce end-expiratory lung volume and improve exercise tolerance, health status and lung function at 6-12 months following treatment, providing an important option in the treatment spectrum between medication therapy and the more invasive surgical options like lung volume reduction surgery or lung transplantation.
“This GOLD rating is very important because it confirms that the body of evidence supporting endobronchial valves is significant and supports the use of this intervention as standard of care for patients suffering from severe emphysema, a form of COPD. As a physician who treats these patients, the Zephyr Valves are the first FDA-approved minimally-invasive option we have had to help patients breathe easier once optimal medical therapy is no longer effective in controlling their symptoms,” said Dr. Gerard Criner, Professor of Medicine and Chair of the Department of Thoracic Surgery and Medicine at Temple University. “Before Zephyr Valves were approved, the only option was major surgery which comes with significant risks. With significant evidence now in place for endobronchial valve treatment, we can now offer patients a choice in treatment options.”
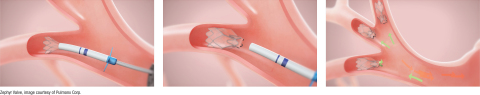
Bronchoscopic lung volume reduction with the Zephyr Valve is a one-time procedure performed through a bronchoscope, which requires no cutting or incisions. During the procedure, an average of four valves are placed in the airways to block off a diseased portion of the lung, which is thereby reduced in size. Reducing hyperinflation and preventing air from getting trapped in the diseased parts of the lung allows the healthier lung tissue to expand and take in more air. This results in patients being able to breathe more easily and experience less shortness of breath. 2 Many patients treated with the Zephyr Valves have reported immediate relief and the ability to go back to doing everyday tasks with greater ease within weeks of treatment.
“GOLD is globally respected for providing in-depth evidence reviews and recommendations that impact patient treatment around the world. We are very pleased to see GOLD give endobronchial valves, like our Zephyr Valve, an evidence “A” rating after a thorough review of published clinical data, which includes multiple Zephyr Valve randomized clinical trials reported in leading medical journals,” said Glen French, President and Chief Executive Officer of Pulmonx. “This level of evidence is an important factor for providers and payers who are seeking to deliver the best treatment options for patients with severe emphysema, a severe form of COPD.”
More on the Zephyr Valve
The Zephyr Valve was granted breakthrough status and approved by the FDA in June 2018, because according to the FDA it “represents a breakthrough technology as the device offers bronchoscopic lung volume reduction without surgery and its associated risks. This device offers a significant, clinically meaningful advantage over the current standard of care and therefore, its availability is also in the best interest of patients.”3 Since 2007 more than 15,000 patients have been treated with the Zephyr Valve worldwide. The Zephyr Valve treatment is recommended at the same level as lung volume reduction surgery in the UK’s National Institute for Health and Care Excellence (NICE) Guidance on the managed of COPD and the GOLD strategy document for the management of COPD.
More about COPD and Emphysema
COPD is a progressive, life-threatening lung disease that includes emphysema and chronic bronchitis. More than 65 million people suffer with COPD globally and it is estimated that 3.2 million deaths were caused by the disease in 2015 (5% of all deaths globally).4 5 Despite taking the best available medications, many COPD and emphysema patients suffer symptoms of hyperinflation, where air becomes trapped in the lungs and prevents fresh air from entering the lungs and thereby causing severe shortness of breath. As a result, patients experience shortness of breath, gradually losing their ability to engage in the most basic daily activities such as climbing a flight of stairs, walking or showering. There are few treatment options for most patients with emphysema and there is no cure. Until now, beyond medication therapy, the only other options for these patients were highly invasive treatments such as lung volume reduction surgery or lung transplantation.
About Pulmonx
Pulmonx Corporation is a medical device company that provides minimally-invasive solutions to treat patients with severe emphysema, a form of COPD. Pulmonx solutions include the Zephyr Endobronchial Valve, a unique minimally-invasive treatment option, and the Chartis Pulmonary Assessment System and the StratX Lung Analysis Platform, a set of innovative assessment tools that enable patient selection and treatment planning. Pulmonx has a compelling body of clinical evidence based on the evaluation of approximately 1,000 patients in multiple randomized controlled clinical studies demonstrating significant improvements in pulmonary function, exercise capacity, dyspnea and quality of life. In June 2018, Pulmonx received pre-market approval, or PMA, through the FDA’s ‘breakthrough device’ pathway to commercialize our Zephyr Valve. Pulmonx solutions are commercially available in more than 25 countries with over 15,000 patients treated. For more information, visit www.MyLungsMyLife.com
GOLD 2020 Report: https://goldcopd.org/gold-reports/
P0894EN_A November
1 Global Strategy for Diagnosis, Management and Prevention of COPD 2020. Retrieved from: https://goldcopd.org/gold-reports/
2 Criner G. et al. Am J Respir Crit Care Med. 2018; 198 (9):1151–1164.
3 PMA P180002: FDA Summary of Safety and Effectiveness Data. June 29, 2018. https://www.accessdata.fda.gov/cdrh_docs/pdf18/P180002B.pdf.
4 Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 2016; 388: 1459-1544.
5 The World Health Organization: Burden of COPD. Accessed November 2019: https://www.who.int/respiratory/copd/burden/en/