IRVINE, Calif.--(BUSINESS WIRE)--Endologix, Inc. (Nasdaq: ELGX), a developer and marketer of innovative treatments for aortic disorders, today announced a response to the U.S. Food and Drug Administration (FDA) update regarding Type III endoleaks with the AFX Endovascular AAA System.
Dr. Matt Thompson, Chief Medical Officer of Endologix Inc. commented, “On September 28, 2017, the FDA issued a letter to health care professionals (“HCPs”) regarding Type III endoleaks after endovascular aneurysm repair (“EVAR”). On June 19, 2018, the FDA issued an update to this letter to reinforce awareness of Type III endoleaks with Endologix’s AFX Strata System. Subsequently, on October 28, 2019, the FDA issued a further update as an FDA Safety Communication in response to an abstract from the Kaiser Integrated Health System, published in the Journal of the American College of Surgeons (Rothenberg et. al.). In this most recent update, the FDA notes that the data included in the abstract suggest that there may be a higher than expected risk of Type III endoleaks occurring with the use of AFX with Duraply and AFX2 endovascular grafts.”
Thompson continued, “The information from Rothenberg et. al, contains retrospectively collected data on 603 patients treated with the AFX Systems between 2011 and 2017 who had a 2.5% cumulative Type III endoleak rate at 2 years. The objectives of the study were described as to provide crude mid-term postoperative outcomes with the Endologix AFX/AFX2 devices. The FDA noted limitations with the data from Rothenberg et. al.in its October 28th update, including the small number of patients implanted with AFX2 (32 patients), the results not being stratified by Type IIIa and Type IIIb endoleaks, and the lack of a comparison of these results to the results from other endovascular graft systems. In our view, the small number of patients with AFX2 at 2 years (13 patients) prevents any clinically meaningful interpretation of these findings. The FDA update provides certain recommendations for patients implanted with AFX, including the need to undergo lifelong yearly surveillance, a practice that is already detailed in the AFX System’s instructions for use as well as in Endologix’s 2016 AFX safety notice and is standard practice with all aortic endografts.”
John Onopchenko, Chief Executive Officer of Endologix Inc. commented, “Our current commercially available versions of the AFX System: the AFX DuraplyTM and AFX2TM products, are manufactured using a different ePTFE processing methodology than AFX Strata and include additional product improvements. Endologix has a robust approach to monitoring the performance of our products through the use of multiple data sets that are benchmarked, compared against other commercially available endografts, and analyzed for concordance. These data include the LEOPARD trial (the only randomized controlled trial of EVAR providing Level 1 evidence), real world data from a vascular registry, our benchmarked MDR complaint data, and meta-analyses of current literature. All of the data sets we use are concordant and demonstrate, through the prospective analysis of 62,461 patients with the AFX system, that our AFX Duraply and AFX2 Systems currently achieve patient outcomes equivalent to other contemporary commercialized endografts, when assessed on an encompassing spectrum of clinical complications.”
Mr. Onopchenko continued, “These data also support our conclusion that changes to the ePTFE manufacturing process from Strata to Duraply, along with the AFX IFU updates, are associated with a reduction in the occurrence of Type III endoleaks for the AFX System. This finding is different from the Rothenberg et. al presentation at the American College of Surgeons, but we regard our datasets as being robust, prospectively collected, and with active contemporary comparators to set the results into appropriate context. We are committed to data transparency, and the data pertaining to our current analysis of the AFX System may be found in our Annual Clinical Updates, which the FDA has reviewed and subsequently referenced in its update: https://endologix.com/wp-content/uploads/2019/10/MM2165-Rev-01-Endologix-2016-2019-AFX-Clinical-Update.pdf. In particular, pages 33-48 detail the most recent results of the LEOPARD study, in which there are no Type IIIb endoleaks with the AFX2 system and no clinically meaningful difference in aggregated patient outcomes when compared to other commercial endografts. We remain confident that the AFX2 device is a safe and effective product when used as indicated, and we will work diligently to understand the outcomes presented by Rothenberg et. al as part of our continuing commitment to ensure that the interests of all EVAR patients, including those implanted with the AFX System, are being prioritized and addressed.”
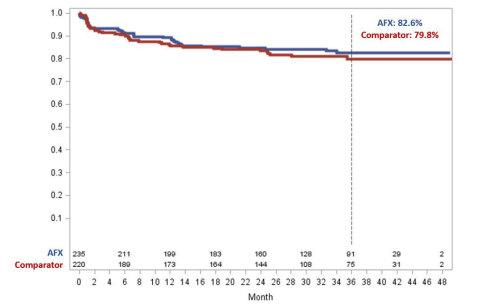
Figure 1 demonstrates freedom from aneurysm related complications (ARC) in the LEOPARD trial. The LEOPARD trial is the only randomized controlled trial of EVAR devices that randomized 455 patients to receive AFX Duraply / AFX2 (235 patients) or contemporary comparator grafts (220 patients). The long-term performance of the AFX/AFX2 Bifurcated (with the DURAPLY ePTFE graft) is supported with 82.6% of subjects being free from ARC at 3-Years compared to the comparator group results of 79.8%. Aneurysm-Related Complications (ARC), is a composite of the most relevant EVAR-related outcomes. These included: peri-operative death (≤30 days), aneurysm rupture, conversion to open surgical repair, post-operative endoleaks, endograft migration (≥10mm), aneurysm enlargement (≥5mm compared to 1-month CT), endograft occlusion, and any reinterventions for device- or aneurysm-related complications.
About Endologix, Inc.
Endologix, Inc. develops and manufactures minimally invasive treatments for aortic disorders. The Company's focus is endovascular stent grafts for the treatment of abdominal aortic aneurysms (AAA). AAA is a weakening of the wall of the aorta, the largest artery in the body, resulting in a balloon-like enlargement. Once AAA develops, it continues to enlarge and, if left untreated, becomes increasingly susceptible to rupture. The overall patient mortality rate for ruptured AAA is approximately 80%, making it a leading cause of death in the United States. For more information, visit www.endologix.com.
Cautions Regarding Forward-Looking Statements
Except for historical information contained herein, this press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements generally can be identified by the use of words such as “anticipate,” “expect,” “could,” “may,” “will,” “believe,” “estimate,” “forecast,” “goal,” “project,” "continue," "outlook," “guidance,” "future,” other words of similar meaning and the use of future dates. Forward-looking statements used in this press release include, but are not limited to, statements regarding the advisability of AFX patients undergoing at least yearly, life-long follow-up, and Endologix’s intent to work diligently to understand the presented AFX data (Rothenberg et. al.) as part of its commitment to prioritize EVAR patients, the accuracy of which are necessarily subject to risks and uncertainties that may cause future events to differ materially and adversely from the statements contained herein. Some of the potential risks and uncertainties that could cause actual results to differ materially and adversely include: future availability and sufficiency of data supporting the benefits of EVAR, generally, and Endologix’s AFX Duraply and AFX2 products, specifically; potential future adverse data or other findings regarding the AFX Strata, AFX Duraply and AFX2 products or other Endologix products; regulatory risks, incuding regulatory risks related to AFX product approvals and regulatory review and obtaining and maintaining approval of these and other other Endologix products in our current and intended markets; risks regarding conduct of Endologix’s clinical trials, registries and other studies and the results thereof; reputational and related commercial risks arising out of potentially adverse data and perceptions regarding Endologix products and technologies; and operational and financial risks that may arise as the result of any adverse findings regarding Endologix’s products, or EVAR generally (including potential loss of sales, and difficulties in accessing the capital markets on acceptable terms or at all). Undue reliance should not be placed upon the forward-looking statements contained in this press release, which speak only as of the date of this press release. Endologix undertakes no obligation to update any forward-looking statements contained in this press release to reflect new information, events or circumstances after the date they are made, or to reflect the occurrence of unanticipated events. Please refer to Endologix's filings with the Securities and Exchange Commission including its Annual Report on Form 10-K for the year ended December 31, 2018 and subsequent Quarterly Reports on Form 10-Q, for more detailed information regarding these risks and uncertainties and other factors that may cause actual results to differ materially from those expressed or implied.
# # #