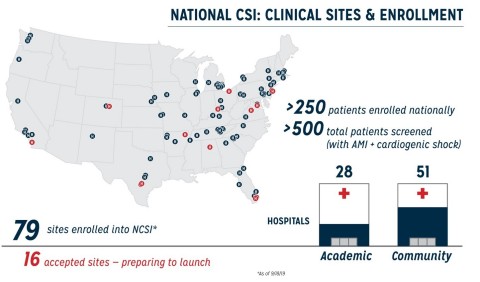
SAN FRANCISCO--(BUSINESS WIRE)--Data presented today from the National Cardiogenic Shock Initiative Study (NCSI) on 250 consecutive AMI cardiogenic shock (AMICS) patients from 49 sites demonstrates 72% survival at discharge with 98% native heart recovery. The patients were treated with the NCSI protocol, which includes placing Abiomed’s (NASDAQ: ABMD) Impella heart pump before revascularization via percutaneous coronary intervention (PCI). The study demonstrates the protocol-based approach to increasing survival rates in cardiogenic shock is reproducible in academic and community hospitals across the United States. Results were presented by William O’Neill, MD, medical director of the Center for Structural Heart Disease at Henry Ford Hospital, during the 31st Transcatheter Cardiovascular Therapeutics (TCT) conference in San Francisco.
Prior to the NCSI, cardiogenic shock survival rates had stagnated for the last 20 years at around 50%. The investigators of the physician-led NCSI are implementing protocols to increase survival and achieve native heart recovery.
The NCSI protocols are derived from best practices that include identifying cardiogenic shock early, minimizing the use of inotropes and placing Impella CP prior to revascularization (PCI). The evidence supporting the best practice of placing Impella pre-PCI comes from thousands of patients in the Impella IQ Database, cVAD Study, Hannover Registry and Detroit Cardiogenic Shock Initiative.
Investigators now plan to institute new escalation protocols that will be applied in the cath lab immediately after Impella-supported PCI in order to further increase patient survival and native heart recovery. The escalation protocols are based on learnings from the Cardiogenic Shock Working Group, the cVAD Study and initial NCSI data.
Specifically, the escalation protocols are:
- After Impella-supported PCI, if the patient is on one inotrope and has a cardiac power output (CPO) of less than 0.8 watts, escalate to Impella 5.0 within 6 hours
- After Impella-supported PCI, if the patient is in right ventricular (RV) dysfunction, quickly escalate to Impella RP or provide oxygenation
“By quickly identifying and escalating patients who need additional support, I believe we can increase cardiogenic shock survival rates to 75%,” said William O’Neill, MD, medical director of the Center for Structural Heart Disease at Henry Ford Hospital. “Physicians around the country have already demonstrated that use of a standardized protocol that includes early placement of the Impella heart pump can dramatically increase survival and native heart recovery. These new escalation protocols can help even more patients return home to their families.”
Since receiving FDA approval, Abiomed has collected data on nearly 100% of U.S. Impella patients in the observational IQ Database. This clinical data, combined with the FDA post-approval studies embedded in Abiomed’s prospective cVAD Study, helped identify and validate best practices for Impella use associated with improved survival and native heart recovery.
“Cardiogenic shock is a state of critical end organ hypoperfusion and unloading with Impella is an FDA approved therapy for patients,” said Seth Bilazarian, MD, the chief medical officer of Abiomed. “Protocols derived from the real-world evidence collected in multiple registries and studies demonstrate unloading with Impella prior to PCI is a crucial component to improvement in survival.”
Impella has the highest level of FDA approval as a therapy for cardiogenic shock to reduce ventricular work and provide the circulatory support necessary to allow heart recovery and early assessment of residual myocardial function.
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are U.S. FDA PMA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5™ with Smart Assist® are U.S. FDA approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart. The Impella RP® is U.S. FDA approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery. Impella is the most studied mechanical circulatory support device in the history of the FDA with real world clinical data on more than 100,000 patients and more than 550 peer-reviewed publications.
In Europe, the Impella 2.5, Impella CP and Impella CP with SmartAssist are CE marked for treatment of high-risk PCI and AMI cardiogenic shock patients for up to 5 days. Impella 5.0 and Impella LD are CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 10 days. The Impella 5.5™ with Smart Assist® is CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 30 days. The Impella RP is CE marked to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, open-heart surgery, or refractory ventricular arrhythmia.
To learn more about the Impella platform of heart pumps, including their approved indications and important safety and risk information associated with the use of the devices, please visit www.impella.com.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support. Our products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart. For additional information, please visit www.abiomed.com.
Abiomed, Impella, Impella 2.5, Impella 5.0, Impella LD, Impella CP, Impella RP, and Impella Connect are registered trademarks of Abiomed, Inc., and are registered in the U.S. and certain foreign countries. Impella BTR, Impella 5.5, Impella ECP, CVAD Study, and SmartAssist are pending trademarks of Abiomed, Inc.
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including statements regarding development of Abiomed's existing and new products, the company's progress toward commercial growth, and future opportunities and expected regulatory approvals. The company's actual results may differ materially from those anticipated in these forward-looking statements based upon a number of factors, including uncertainties associated with development, testing and related regulatory approvals, including the potential for future losses, complex manufacturing, high quality requirements, dependence on limited sources of supply, competition, technological change, government regulation, litigation matters, future capital needs and uncertainty of additional financing, and other risks and challenges detailed in the company's filings with the Securities and Exchange Commission, including the most recently filed Annual Report on Form 10-K and Quarterly Report on Form 10-Q. Readers are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. The company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this release or to reflect the occurrence of unanticipated events.