SAN FRANCISCO--(BUSINESS WIRE)--Abiomed (NASDAQ: ABMD) announces the results of PROTECT III, the ongoing, prospective, single-arm FDA post-approval study for the PMA approval of Impella 2.5 and Impella CP in high-risk PCI. PROTECT III follows the PROTECT II Randomized Controlled Trial (RCT).
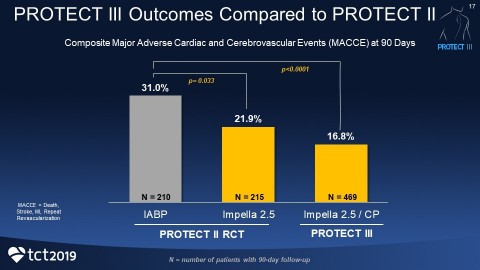
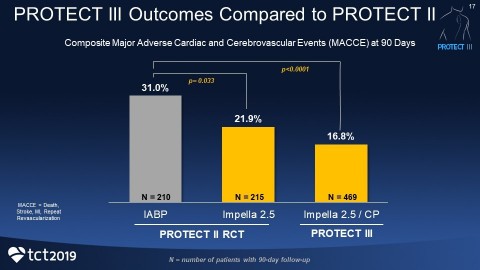
PROTECT III demonstrates a reduction in the primary endpoint of death, stroke, myocardial infarction and repeat procedures at 90 days with Impella-supported Protected PCI, compared to PROTECT II. Death, stroke, myocardial infarction and repeat procedures are known collectively as major adverse cardiac and cerebrovascular events (MACCE).
The findings of this interim analysis on 898 patients were announced today at the 31st Transcatheter Cardiovascular Therapeutics (TCT) conference by a member of the study’s steering committee, Jeffrey J. Popma, MD, the director of interventional cardiology clinical services at Beth Israel Deaconess Medical Center, and professor of medicine at Harvard Medical School.
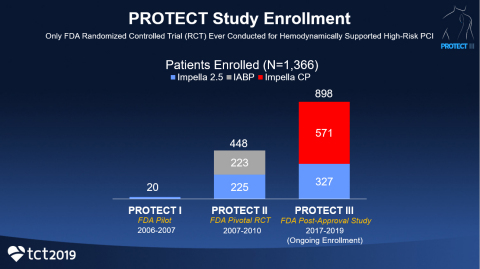
The PROTECT series of FDA clinical studies, which includes PROTECT I, the PROTECT II RCT and PROTECT III, is the largest-ever FDA study of hemodynamically supported high-risk PCI patients. The series has enrolled 1,366 patients as of July and includes the only FDA randomized controlled trial ever conducted for hemodynamically supported high-risk PCI.
Protect III Findings Announced
Patients in PROTECT III are statistically older (71 years), include more women (26%) and non-Caucasians (33%), received longer support and had more complex procedures with more vessels treated than patients in PROTECT II. Yet the 90-day MACCE rate in PROTECT III is lower than the intra-aortic balloon pump (IABP) control arm from PROTECT II. The composite MACCE rate in the IABP arm was 31%, compared to the Impella 2.5 and Impella CP arm at 16.8% (p<0.0001).
“Based on the learnings from the PROTECT studies, decisions to provide hemodynamic support during PCI should be made in the context of providing complete revascularization and patient outcomes should include in-hospital and out-of-hospital improvements,” said Dr. Popma. “Prior studies have demonstrated it is important to achieve complete revascularization because it can result in a 30-50% reduction in MACCE, compared to incomplete revascularization.”
Summary of Acute Kidney Injury (AKI) Data
High-risk PCI patients face elevated risk of AKI due to high levels of contrast and long procedures. Additionally, chronic kidney disease is a common co-morbidity. According to a NCDR Cath PCI Registry Study of 985,737 patients, in-hospital mortality for patients with AKI is 10% and escalates to 34% if dialysis is required.
In PROTECT III, a sub-study (N=106) of patients were evaluated for acute kidney injury (AKI) rate and compared to a propensity matched control group (N=106). The results will be presented during the TCT Scientific Sessions. Multiple studies with Impella have measured the AKI rate based on predicted AKI risk factors, including contrast volume. These include:
- Circulation, 2012 -The PROTECT II RCT found Impella patients had statistically higher contrast volume than the IABP arm (267 ml vs. 241 ml, p=0.04), but a numerically lower rate of acute renal dysfunction at 30 days and 90 days (p=0.792 and p=0.776, respectively), showing a decoupling of contrast volume from renal dysfunction
- Circulation Research, 2017 - Flaherty, et al., identified a six-fold reduction in AKI requiring dialysis when Impella support was used, compared to without Impella support (p=0.031).
- Catherization and Cardiovascular Interventions, 2019 – Flaherty et al., found, compared to a predicted rate of 22% (using the Mehran risk score), only 5% of Impella-supported patients developed AKI (exclusively stage 1) at 48 hours, representing a 77.6% lower AKI risk (p<0.0001).
The PROTECT Series
The PROTECT III Study includes 898 patients enrolled at 45 sites in the United States between March 2017 and July 2019. The PROTECT Series now includes 1,366 patients and Impella is the most studied mechanical circulatory support device in the history of the FDA.
“The totality of clinical data in favor of Impella supported high-risk PCI allows interventional cardiologists to be confident they are using the optimal treatment and technologies to help achieve complete revascularization in a single setting, improve procedural hemodynamic stability and improve patient quality of life,” said William O’Neill, MD, medical director of the Center for Structural Heart Disease at Henry Ford Hospital.
Abiomed initiated the PROTECT series of studies in 2006 with PROTECT I and conducted the PROTECT II RCT from 2007 to 2010. In 2009, Abiomed created the Impella Quality (IQ) Database that has now collected real world clinical data on more than 100,000 patients.
The FDA granted Impella its highest level of safety and efficacy approval, a post-market approval (PMA) for a first-of-its-kind indication for high-risk PCI, based on the results of PROTECT I (N=20), the PROTECT II RCT (N=448) and registry data. Impella is included in eight clinical guidelines and more than 550 peer-reviewed publications.
A video of Dr. Popma commenting on the results of PROTECT III and slides from Dr. Popma’s presentation at TCT are available online at www.ProtectIIIStudy.com. Dr. Popma’s presentation from TCT will be posted at www.ProtectIIIStudy.com within the next 24 hours.
The study is sponsored by Abiomed as part of its commitment to improving clinical outcomes.
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are U.S. FDA PMA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5™ with Smart Assist® are U.S. FDA approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart. The Impella RP® is U.S. FDA approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery. Impella is the most studied mechanical circulatory support device in the history of the FDA with real world clinical data on more than 100,000 patients and more than 550 peer-reviewed publications.
In Europe, the Impella 2.5, Impella CP and Impella CP with SmartAssist are CE marked for treatment of high-risk PCI and AMI cardiogenic shock patients for up to 5 days. Impella 5.0 and Impella LD are CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 10 days. The Impella 5.5™ with Smart Assist® is CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 30 days. The Impella RP is CE marked to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, open-heart surgery, or refractory ventricular arrhythmia.
To learn more about the Impella platform of heart pumps, including their approved indications and important safety and risk information associated with the use of the devices, please visit www.impella.com.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support. Our products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart. For additional information, please visit www.abiomed.com.
Abiomed, Impella, Impella 2.5, Impella 5.0, Impella LD, Impella CP, Impella RP, and Impella Connect are registered trademarks of Abiomed, Inc., and are registered in the U.S. and certain foreign countries. Impella BTR, Impella 5.5, Impella ECP, CVAD Study, and SmartAssist are pending trademarks of Abiomed, Inc.
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including statements regarding development of Abiomed's existing and new products, the company's progress toward commercial growth, and future opportunities and expected regulatory approvals. The company's actual results may differ materially from those anticipated in these forward-looking statements based upon a number of factors, including uncertainties associated with development, testing and related regulatory approvals, including the potential for future losses, complex manufacturing, high quality requirements, dependence on limited sources of supply, competition, technological change, government regulation, litigation matters, future capital needs and uncertainty of additional financing, and other risks and challenges detailed in the company's filings with the Securities and Exchange Commission, including the most recently filed Annual Report on Form 10-K and Quarterly Report on Form 10-Q. Readers are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. The company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this release or to reflect the occurrence of unanticipated events.