AUSTIN, Texas--(BUSINESS WIRE)--Savara Inc. (Nasdaq: SVRA), an orphan lung disease company, today announced top line data from IMPALA, a pivotal Phase 3 clinical study evaluating Molgradex, an inhaled formulation of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF), for the treatment of aPAP. 138 patients were randomized and received treatment for 24 weeks in one of three arms: 1) Molgradex 300 µg administered once daily continuously over 24 weeks, 2) Molgradex 300 µg, and matching placebo, administered once daily in 7-day intermittent cycles of each, or 3) inhaled placebo administered once daily continuously over 24 weeks.
Primary Endpoint in the Intention-to-Treat (ITT) Population
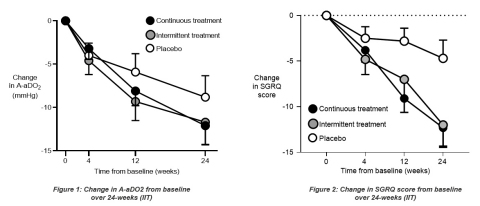
An average A-aDO2 improvement of 12.1 mmHg was observed in the continuous dosing arm, compared to an average A-aDO2 improvement of 8.8 mmHg in the placebo arm. With an estimated 4.6 mmHg treatment difference (p=0.17), the study did not meet its primary endpoint.
Secondary Endpoints in the ITT Population
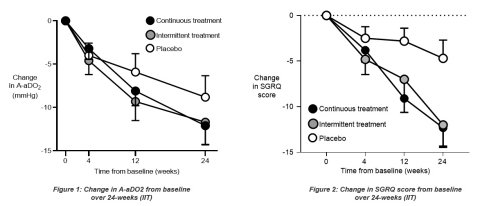
An average improvement of 12.3 points in the SGRQ (a patient-reported outcomes/quality-of-life measure) was observed in the continuous dosing arm compared to an average improvement of 4.7 points in the placebo arm. The estimated treatment difference was 7.6 points (p=0.01) which was statistically significant and approximately two times the generally accepted clinically meaningful difference of four points for this measure.
The other two key secondary endpoints, the six-minute walk distance (6MWD) and requirement for whole lung lavage (WLL), were numerically in favor of the continuous dosing arm, but the differences to placebo were not statistically significant. Patients in the continuous dosing arm showed a mean improvement of 39.6 meters in the 6MWD, while the intermittent and placebo arms showed improvements of 11.3 meters and 6.0 meters, respectively. Four patients in each of the active arms, and six patients in the placebo arm underwent a WLL during the treatment period. Given that some patients received more than one WLL, the total number of WLLs observed in the continuous dosing arm was 9, with 7 observed in the intermittent dosing arm and 17 in the placebo arm.
In addition to the A-aDO2, the diffusing capacity of the lungs for carbon monoxide (DLCO) was assessed as a secondary endpoint to evaluate the efficacy of Molgradex on gas transfer. Patients in the continuous dosing arm showed a mean improvement of 11.6% predicted in DLCO, whereas the intermittent dosing and placebo arms showed a 7.7% predicted and 3.9% predicted improvement, respectively. The estimated treatment difference of 7.9% predicted (p=0.007) between the continuous dosing arm and placebo was statistically significant.
“Taken together, the trends in reduction of A-aDO2, as well as the improvement in the diffusion capacity, are very consistent with reduction of the surfactant burden that causes the clinical symptoms of aPAP,” said Bruce Trapnell, M.D., Lead Investigator of the IMPALA study in the U.S. and Professor of Medicine and Pediatrics, University of Cincinnati College of Medicine. “Most importantly, the impressive improvement in quality of life, as measured by the SGRQ, suggest that Molgradex not only improved objective measures of oxygenation, but also had a clinically meaningful therapeutic effect. I believe these data demonstrate that Molgradex can become an important pharmacological treatment option for patients with aPAP.”
Safety and Tolerability in the ITT Population
The number of treatment emergent adverse events (TEAEs), including serious adverse events, occurred with similar frequency and severity in the active arms compared to placebo. The number of subjects discontinuing due to TEAEs was as follows: continuous dosing arm; n=2, intermittent dosing arm; n=1, placebo arm; n=1.
The Company plans to meet with the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) in the coming months to discuss these data and the path forward.
"Disappointingly, with the placebo effect stronger than anticipated, the study did not meet its primary endpoint,” said Rob Neville, Chief Executive Officer, Savara. “However, we remain encouraged about the results of IMPALA, most notably the significant improvement in SGRQ, the consistency of trends and improvements seen across the endpoints and the favorable safety profile. We are preparing to meet with the FDA and EMA to discuss the results from this study and to determine our options to seek approval based on the current data, and potentially conduct an additional study incorporating the learnings from IMPALA. It is with much gratitude that we acknowledge the patients participating in the study. It is on their behalf that we will continue to pursue our goal of bringing this important therapy to market.”
The Company intends to submit the full data set from IMPALA to a peer-reviewed scientific journal or to an upcoming medical meeting for consideration.
Conference Call and Webcast
The Company will host a conference call today at 5:30 p.m. Eastern Time (ET) / 2:30 p.m. Pacific Time (PT) to discuss these results. Shareholders and other interested parties may access the conference call by dialing (855) 239-3120 from the U.S., (855) 669-9657 from Canada, and (412) 542-4127 from elsewhere outside the U.S. and request the “Savara Inc.” call. A live webcast of the conference call will be available online in the Investors section of Savara’s website at https://www.savarapharma.com/investors/events-presentations/.
Approximately one hour after the call, a replay of the webcast will be available on Savara’s website for 30 days, and a telephone replay will be available through June 19, 2019 by dialing (877) 344-7529 from the U.S., (855) 669-9658 from Canada and (412) 317-0088 from elsewhere outside the U.S. and entering the replay access code 10132445.
About the IMPALA Phase 3 Clinical Study
The IMPALA study is a randomized, double-blind, placebo-controlled study designed to compare the efficacy and safety of Molgradex with placebo in patients with aPAP. The study is being conducted in 18 countries worldwide. Patients were randomized to receive treatment for 24 weeks in one of three arms: 1) Molgradex 300 µg administered once daily continuously over 24 weeks, 2) Molgradex 300 µg, and matching placebo, administered once daily in 7-day intermittent cycles of each, or 3) inhaled placebo administered once daily continuously over 24 weeks. At the end of the 24-week double-blind period, all treatment arms roll into a 24-week open-label follow-on period and receive Molgradex 300 ug administered daily in 7-day intermittent cycles. The primary endpoint in the study is the absolute change from baseline in A-aDO2, a measure of the patient’s oxygenation status, following 24 weeks of treatment. In addition, the FDA will focus its review on three key secondary endpoints to evaluate improvement in clinical symptoms and function, including the 6MWD, SGRQ, and the time to / requirement for whole lung lavage.
About Savara
Savara is an orphan lung disease company. Savara’s pipeline comprises Molgradex, an inhaled granulocyte-macrophage colony-stimulating factor (GM-CSF) in Phase 3 development for autoimmune pulmonary alveolar proteinosis (aPAP), in Phase 2a development for nontuberculous mycobacterial (NTM) lung infection in both non-cystic fibrosis (CF) and CF-affected individuals with chronic NTM lung infection; and AeroVanc, a Phase 3-stage inhaled vancomycin for treatment of persistent methicillin-resistant Staphylococcus aureus (MRSA) lung infection in CF. Savara’s strategy involves expanding its pipeline of potentially best-in-class products through indication expansion, strategic development partnerships and product acquisitions, with the goal of becoming a leading company in its field. The most recent acquisition is aerosolized amikacin/fosfomycin, a Phase 2-ready combination antibiotic for which the initial indication will focus on non-CF bronchiectasis patients with chronic lung infection and frequent exacerbations. Savara’s management team has significant experience in orphan drug development and pulmonary medicine, identifying unmet needs, developing and acquiring new product candidates, and effectively advancing them to approvals and commercialization. More information can be found at www.savarapharma.com. (Twitter: @SavaraPharma, LinkedIn: www.linkedin.com/company/savara-pharmaceuticals/)
Forward-Looking Statements
Savara cautions you that statements in this press release that are not a description of historical fact are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of words referencing future events or circumstances such as “expect,” “intend,” “plan,” “anticipate,” “believe,” and “will,” among others. Such statements include, but are not limited to, Dr. Trapnell’s statements regarding trends in reduction of A-aDO2 as well as the improvement in the diffusion capacity being very consistent with reduction of the surfactant burden that causes the clinical symptoms of aPAP, the impressive improvement in quality of life, as measured by the SGRQ, suggest that Molgradex not only improved objective measures of oxygenation, but also had a clinically meaningful therapeutic effect and the belief that Molgradex can become an important pharmacological treatment option for patients with aPAP, statements related to Savara’s plans to meet with the FDA and EMA to discuss the IMPALA data, including the path forward and to determine our options to seek approval based on the current data and potentially conduct an additional study incorporating the learnings from IMPALA, that we remain encouraged by the results of the IMPALA study, most notably the significant improvement in SGRQ, the consistency of the trends and improvements seen across the endpoints and the favorable safety profile, that we will continue to pursue our goal of bringing this important therapy to market, that Savara intends to submit the full data set from IMPALA to a peer-reviewed scientific journal or to an upcoming medical meeting for consideration, and Savara’s strategy. Savara may not actually achieve any of the matters referred to in such forward-looking statements, and you should not place undue reliance on these forward-looking statements. These forward-looking statements are based upon Savara’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with the outcome of our ongoing and planned clinical trials for our product candidates, updated or refined data based on the continuing review and analysis of the IMPALA data, the outcome of our planned meetings with the FDA and EMA to discuss the IMPALA data and the path forward, the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient resources for Savara’s operations and to conduct or continue planned clinical development programs, the ability to obtain the necessary patient enrollment for our product candidates in a timely manner, the ability to successfully identify product acquisition candidates, the ability to successfully develop our product candidates, the risks associated with the process of developing, obtaining regulatory approval for and commercializing drug candidates such as Molgradex that are safe and effective for use as human therapeutics and the timing and ability of Savara to raise additional equity capital as needed to fund continued operations. All forward-looking statements are expressly qualified in their entirety by these cautionary statements. For a detailed description of our risks and uncertainties, you are encouraged to review our documents filed with the SEC including our recent filings on Form 8-K, Form 10-K and Form 10-Q. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they were made. Savara undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as may be required by law.