CARLSBAD, Calif. & SARASOTA, Fla.--(BUSINESS WIRE)--Lumos Diagnostics, a California-based full-service point-of-care (POC) diagnostic development company and previous spinoff from Australia-based Planet Innovation and RPS Diagnostics, a Florida-based commercial diagnostic developer, manufacturer, and marketer of POC diagnostic tests, announced today that they have merged. The combined company will be called Lumos Diagnostics.
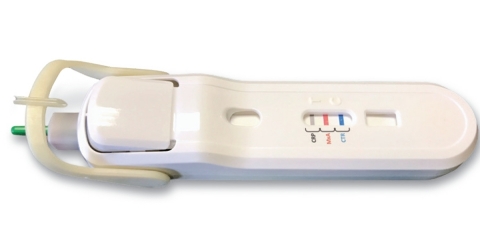
Lumos Diagnostics’ initial focus is on the international launch of the FebriDx® test, a rapid, in-office POC test that incorporates a built-in safety lancet to obtain a fingerstick blood sample, rotating blood collection and transfer system, and integrated push-button buffer activation, to provide clinicians with a rapid assessment of the body’s immune response to an acute respiratory infection (ARI). The single use test identifies patients within 10 minutes that have a clinically significant underlying infection and aids in the differentiation of viral and bacterial ARIs through the simultaneous detection of both Myxovirus resistance protein A (MxA) and C-reactive protein (CRP) directly from peripheral whole blood. MxA is an intracellular protein that becomes elevated in the presence of acute viral infection and CRP is an acute-phase inflammatory protein that is elevated in the presence of clinically significant infection.
Sam Lanyon, chairman of the board for Lumos Diagnostics states, “We have evaluated many technologies over the years, and believe that combining RPS Diagnostics’ novel biomarker technology and commercial experience together with Lumos Diagnostics’ reader-based platform results in a highly strategic and synergistic union that will support a robust pipeline and commercial success.”
ARI are often highly contagious and result in more than half of all antibiotics that are prescribed for outpatient primary and urgent care visits. ARIs may be associated with nonspecific flu-like symptoms, including fever, sore throat, cough, nasal congestion, and fatigue. Bacterial infections pose the highest risk of morbidity and are the only infections that benefit from antibiotic treatment. Diagnostic uncertainty from overlapping ARI symptoms and signs combined with patient or parent pressures for antibiotic prescriptions lead to more than 50% of all unnecessary antibiotic prescriptions. Rapid single pathogen tests, such as for Strep A and Influenza, cannot differentiate colonization from true infection and are indicated for only specific respiratory conditions, accounting for about 15% and 25% of patient visits, respectively; whereas the majority of the patients have other viral infections, bacterial infections, or a microbiologically unconfirmed respiratory illness.
Clinical performance from two prospective multi-center U.S. clinical trials demonstrate the FebriDx test’s high accuracy and 97-99% negative predictive value to exclude a bacterial infection. Moreover, in a small United Kingdom outcome study, FebriDx was shown to alter clinical management decisions in 48% of patients tested and reduced unnecessary antibiotic prescriptions by 80%. By enabling a rapid diagnosis at the initial office visit, the FebriDx test may help to limit the amount of unnecessary antibiotic prescriptions that can lead to avoidable adverse reactions and antibiotic resistance, resulting in lower costs.
Robert Sambursky, MD, the president and chief executive officer of RPS Diagnostics, who will continue in the same role for the joint Lumos Diagnostics entity states, “Using FebriDx to help triage outpatient ARI is a game changer because successful antibiotic stewardship requires the clinician to first rule out a clinically significant bacterial infection. In addition, access to novel Lumos Diagnostics reader technology will facilitate next generation digital enhancements which will accelerate speed to results, allow for quantitation, and enhance objectivity of our branded product lines.”
Lumos Diagnostics
Lumos Diagnostics provides rapid, cost effective, and complete point-of-care (POC) diagnostic test solutions that utilize proprietary digital reader platforms to help healthcare professionals more accurately diagnose and manage diseases and medical conditions. Lumos provides assay development and manufacturing services for customized POC tests as well as directly develops, manufactures, and will commercialize a suite of Lumos-branded POC tests. Conditions targeted by Lumos tests include infectious and inflammatory diseases with unmet diagnostic needs, including fever, biological threats, and infectious disease, resulting in less unnecessary treatments with associated adverse events, reduced spread of disease, and more effective antibiotic stewardship initiatives.
The FebriDx test has received Health Canada approval, Saudi FDA clearance, Singapore Health Sciences Authority registration, and is CE marked for sale in Europe. At this time, the FebriDx test has not received U.S. Food and Drug Administration (FDA) clearance and is not commercially available in the United States. For more information on Lumos Diagnostics, visit lumosdiagnostics.com, and for more information on FebriDx, visit febridx.com.