PARIS & CAMBRIDGE, Mass.--(BUSINESS WIRE)--Regulatory News:
NANOBIOTIX (Paris:NANO) (Euronext: NANO – ISIN: FR0011341205 – the “Company”), a clinical-stage nanomedicine company pioneering new approaches to the treatment of cancer, today announced its unaudited1 consolidated results for the fiscal year ended December 31, 2018:
-
Major milestones achieved during the year:
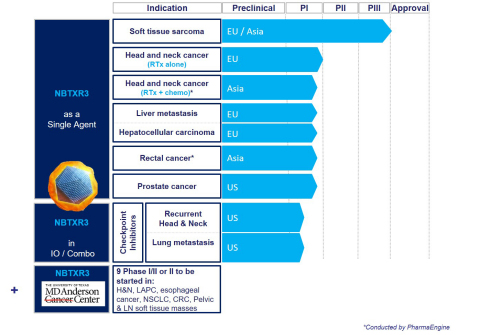
- Positive Phase II/III results for NBTXR3 in patients with locally advanced Soft Tissue Sarcoma demonstrating clinical meaningful benefits versus standard of care
- Positive clinical update from Phase I Head and Neck and Phase I/II Liver cancers trials presented at major congresses
- Completion of Phase I dose escalation in Head and Neck cancers
- Engaged major preclinical collaborations with highly respected U.S. cancer centers including the University of Texas MD Anderson Cancer Center, the Providence Cancer Institute and Weill Cornell Medicine
-
Consolidated cash available of €36.2M at December 31, 2018
- Strengthened by the payment of a €16M first tranche pursuant to a €40M non-dilutive financing agreement established with the European Investment Bank in 2018
- Expenses in R&D proceeding as expected according to clinical development plan
Philippe Mauberna, Chief Financial Officer of Nanobiotix said: “2018 has been a major year for Nanobiotix, we made significant progress in the financial and clinical plan of the company. R&D expenses are linked to our development plan and the hiring of experts in key positions. We are delighted by the positive results in Soft Tissue Sarcoma Phase II/III and the encouraging update from Head and Neck and Liver cancers Phase I/II trials. Indeed, the support of the European Investment Bank strengthens our balance sheet, which allows us to move forward with our 2019 upcoming key milestones.”
The unaudited1 consolidated financial statements for the fiscal year ended December 31, 2018 were approved by the executive board and reviewed by the supervisory board of the Company on March 15, 2019.
Consolidated Income statement (unaudited)1
| K€ | 2018 | 2017 | |||
| Total revenue and other income | 3,479 | 3,722 | |||
|
Sales
Services Other sales Licences
Other revenues Research Tax Credit Subsidies Other |
116
109 7 -
3,363 3,251 90 22 |
252 229 23 -
3,470 3,259 154 57 |
|||
|
Research & Development (R&D) costs (incl. |
(20,893) | (17,733) | |||
|
Selling, General and Administrative (SG&A) |
(12,653) | (11,254) | |||
| Operating loss | (30,066) | (25,267) | |||
| Financial loss | (277) | (876) | |||
| Income tax | - | - | |||
| Net loss for the period | (30,345) | (26,143) |
_______________
1 The Company’s statutory auditors have
completed their audit work on the 2018 financial statements and expect
to issue their audit report on March 20th, 2019.
Financial Review (unaudited)2
Total Revenue in 2018 amounted to €3.5M vs. €3.7M in 2017, mainly due to:
- Revenues related to services provided mainly by the Company to its partner, PharmaEngine, pursuant to a commercial agreement, amounted to €116K in 2018 (vs. €252K in 2017); and
- Other revenues of €3,363K in 2018 (vs. €3,469K in 2017) mainly related to the Research Tax Credit (Crédit d’Impôt Recherche - CIR).
Total Operating expenses reached €33.5M in 2018 vs. €29.0M in 2017:
- R&D expenses (including share-based payments) in 2018 amounted to €20.9M (vs. €17.7M in 2017); the variance comes from an increase in operations (opening of the new production site, launch and extension of new studies) as well as the addition of highly-qualified staff;
- SG&A costs (including share-based payments) in 2018 were €12.7M (vs. €11.3M in 2017).
Total headcount on a consolidated basis reached 102 as of December 31, 2018 vs. 85 as of December 31, 2017, in line with the Company’s growth.
Net loss after tax amounts to €30.3M as of December 31, 2018 (vs. €26.1M loss as of December 31, 2017).
Cash available at December 31, 2018 amounted to €36.2M.
-------------------------------------
Nanobiotix activities and achievements in 2018
Clinical
Positive Phase II/III results for NBTXR3 in patients with locally advanced Soft Tissue Sarcoma demonstrated clinical meaningful benefits versus standard of care
Nanobiotix announced positive results from its Phase II/III clinical trial of NBTXR3 in patients with locally advanced soft tissue sarcoma. The trial achieved its primary endpoint with a pathological complete response rate. It also achieved its secondary endpoint in operability. NBTXR3 demonstrated clinical meaningful benefits for such patients versus standard of care. The data also showed that NBTXR3 was well tolerated. The randomized trial validated the first-in-class mode of action of NBTXR3.
These positive results were presented by Dr. Sylvie Bonvalot at the ESMO and ASTRO annual conferences.
Positive Update on Head and Neck cancers Phase I trial showing potential impact for survival
Nanobiotix provided an update on the Head and Neck Phase I Trial with NBTXR3 data presented at ImmunoRad 2018. The Phase I Trial focus on elderly and frail patients ineligible for cisplatin or intolerant to cetuximab. The data show the potential impact on survival in this patient population.
Encouraging Data from Phase I/II Liver cancers trial
Nanobiotix presented initial promising data from Phase I/II Liver trial evaluating NBTXR3 in liver cancers, including primary (Hepatocellular, HCC) and liver metastasis from other tumors at the American Society of Clinical Gastrointestinal annual meeting (ASCO GI). These positive results show that NBTXR3 was well tolerated with no adverse event related to NBTXR3 and no dose-limiting toxicity.
Collaboration in preclinical research
Collaboration with the Providence Cancer Institute
Nanobiotix partnered with the Providence Cancer Institute to run immunotherapeutic preclinical research in pancreatic cancer. This collaboration will provide essential preclinical data on the ability of NBTXR3 activated by radiotherapy to induce an antitumoral immune response.
_______________
2 The Company’s statutory auditors have
completed their audit work on the 2018 financial statements and expect
to issue their audit report on March 20th, 2019.
The University of Texas MD Anderson Cancer Center and Nanobiotix have an agreement in pre-clinical research
Nanobiotix and the University of Texas MD Anderson Cancer Center have an agreement to run immunotherapeutic pre-clinical research in lung cancer. The main objectives of this project, with one of the world’s leading oncology research centers, is to provide preclinical data using NBTXR3 activated by radiotherapy plus anti PD-1 Nivolumab (murine version of Opdivo™).
Partnering with Weill Cornell Medicine on pre-clinical studies
Nanobiotix and Weill Cornell Medicine partnered to perform pre-clinical studies to evaluate the impact of NBTXR3 on cGAS-STING pathway in mammary cancers. The main objective is to study the impact of NBTXR3 activated by radiotherapy on cGAS-STING, a key component of the anti-tumor immune response. Data generated from this collaboration could provide support for the assertion that NBTXR3 activated by radiotherapy can increase the anti-tumor immune response compared to radiotherapy alone.
Preclinical data showing NBTXR3 can activate cGAS-STING pathway
Nanobiotix presented preclinical data showing NBTXR3 nanoparticles can activate the cGAS-STING pathway at the American Association for Cancer Research (AACR). These observations support the rationale for using NBTXR3 with radiation therapy in combination with immunotherapeutic agents and/or STING agonist to transform tumors into an in-situ cancer vaccine.
Financial events
Launch of a €40M non-dilutive financing agreement with the European Investment Bank
Nanobiotix announced in July 2018, the launching of a non-dilutive financing agreement with the European Investment Bank to boost its research, development and innovation activities. This agreement will allow the Company to borrow up to €40M through loans before July 26, 2020 subject to achieving a set of agreed performance criteria.
In October 2018, Nanobiotix received the first tranche disbursement of €16M. The proceeds will be used to speed up development of lead product NBTXR3 in Head and Neck cancers and to support the European go-to-market strategy.
Selection to the Euronext Tech 40
This honor recognizes the best performing Tech SMEs listed on Euronext markets. An independent group of European experts annually selects 40 great companies on the basis of their business, financial and stock market performance.
Events 2019
Large-scale collaboration on NBTXR3 with the University of Texas MD Anderson Cancer Center
In January 2019, Nanobiotix and the University of Texas MD Anderson Cancer Center announced a large-scale comprehensive clinical collaboration on NBTXR3. The collaboration will initially support nine new Phase I/II clinical trials with NBTXR3 for use in treating six cancer types – head and neck, pancreatic, thoracic, lung, gastrointestinal and genitourinary cancers – and will involve around 340 patients. Most of the trials are expected to be launched in 2019. Nanobiotix will finance at least approximately $11M, a portion of which has been paid as of the start of the collaboration, with additional amounts payable during development and upon specified regulatory milestone.
Announced plans to conduct registered public offering in the US
In January 2019, Nanobiotix announced that it plans to conduct a registered public offering of its ordinary shares, including the form of American Depositary Shares (ADSs) in the United States. Nanobiotix submitted a confidential draft registration statement on Form F-1 to the U.S. Securities and Exchange Commission.
Second tranche disbursement from the European Investment Bank
In March 2019, Nanobiotix received the second tranche of disbursement of €14M from the European Investment Bank. This payment was granted because of the company’s achievement on two criteria: determination of the recommended dose at 22% of the tumor volume for head and neck cancers treatment following the end of Phase I clinical trial with NBTXR3 and positive evaluation of the clinical benefit/risk ratio of NBTXR3 in soft tissue sarcomas Phase II/III by the clinical expert mandated by the French medical device notified body, GMED.
2019 Perspectives
This year, Nanobiotix expects to receive its CE mark for NBTXR3 for the treatment of Soft Tissue Sarcoma, which would improve access to the product for cancer patients.
In parallel, clinical development should advance with the publication of data in several cancer types.
Newsflow (anticipated)
- 2019 – European market approval/CE mark for the treatment of Soft Tissue Sarcoma cancer
- 1H2019 -- Preclinical data regarding immuno-oncology using NBTXR3 in combination with checkpoint inhibitors
- 1H2019 -- FDA feedback on NBTXR3 clinical plan in Head and Neck cancers
- 2H2019 – Presentation of final Head & Neck Phase I dose escalation results
- 2H2019 -- Potential early results in immuno-oncology with anti-PD-1 study
- Multiple launches of clinical trials within MD Anderson collaboration
- Additional news on other clinical trials and preclinical programs
Additional news on plans to conduct registered public offering in the United States
-Ends-
Next financial press release: revenue for Q1 2019 on April 30, 2019
Nanobiotix’ Annual General Meeting will be held on April 11, 2019 at 2:30 pm Pullman Bercy – Saumur Champigny – 1 rue de Libourne 75012 Paris, France.
About NANOBIOTIX: www.nanobiotix.com
Incorporated in 2003, Nanobiotix is a leading, clinical-stage nanomedicine company pioneering new approaches to significantly change patient outcomes by bringing nanophysics to the heart of the cell.
The Nanobiotix philosophy is rooted in designing pioneering, physical-based approaches to bring highly effective and generalized solutions to address unmet medical needs and challenges.
Nanobiotix’s first-in-class, proprietary lead technology, NanoXray, aims to expand radiotherapy benefits for millions of cancer patients. Nanobiotix’s Immuno-Oncology program has the potential to bring a new dimension to cancer immunotherapies.
Nanobiotix is listed on the regulated market of Euronext in Paris (Euronext: NANO / ISIN: FR0011341205; Bloomberg: NANO: FP). The Company’s headquarters are in Paris, France, with a U.S. affiliate in Cambridge, MA, and European affiliates in Spain and Germany.
Disclaimer
This press release contains certain forward-looking statements concerning Nanobiotix and its business. Such forward-looking statements are based on assumptions that Nanobiotix considers to be reasonable. However, there can be no assurance that the estimates contained in such forwardlooking statements will be verified, which estimates are subject to numerous risks including the risks set forth in the reference document of Nanobiotix filed with the French Financial Markets Authority (Autorité des Marchés Financiers) under number D.17-0470 on April 28, 2017 as well as in its 2017 annual financial report filed with the French Financial Markets Authority on March 29, 2018 (a copy of which is available on www.nanobiotix.com) and to the development of economic conditions, financial markets and the markets in which Nanobiotix operates. The forward-looking statements contained in this press release are also subject to risks not yet known to Nanobiotix or not currently considered material by Nanobiotix. The occurrence of all or part of such risks could cause actual results, financial conditions, performance or achievements of Nanobiotix to be materially different from such forward-looking statements. This press release and the information that it contains do not constitute an offer to sell or subscribe for, or a solicitation of an offer to purchase or subscribe for, Nanobiotix shares in any country. At the moment NBTXR3 does not bear a CE mark and is not permitted to be placed on the market or put into service until NBTXR3 has obtained a CE mark.