PARIS--(BUSINESS WIRE)--Affluent Medical (Paris:ALAM), a French medtech company specialized in innovative, minimally invasive implants designed to restore key physiological functions for patients suffering from heart and vascular diseases, as well as urinary incontinence, today announces positive results from its SCOPE 1 clinical trial validating the efficacy of KARDIOZIS technology.
Results have been presented Saturday February 9th, 2019 at Controversies And Updates in Vascular Surgery congress (CACVS) in Paris by Professor Dominique Fabre, MD, principal investigator. Study contributors are Frederic Cochennec, MD, Claude Angel, MD, Eric Allaire, MD, Philippe Brenot, MD, Riyad Bourkaib MD, Jean-Yves Riou, MD, Pascal Desgranges, MD, Benoit Gerardin, MD, Delphine Mitilian MD, Carlos Garcia Alonzo, MD, Sarah Hamdi, MD, Jean-Pierre Becquemin, MD, and Professor Stephan Haulon, MD.
SCOPE 1 clinical trial started in 2013 under the lead of Professor Dominique Fabre, thoracic and vascular surgeon at MARIE LANNELONGUE hospital - Le Plessis-Robinson (France), in collaboration with PARIS-SUD SACLAY University (France), and with the participation of Frederic Cochennec, vascular surgeon at HENRI MONDOR hospital – Creteil (France), in collaboration with PARIS-EST University (France).
SCOPE 1 is a controlled, randomized, prospective, multi-centric clinical trial at evaluating efficacy and clinical outcomes of embolization of the aneurysm sac using thrombogenic fibers when performing a conventional EndoVascular Aneurysm Repair (EVAR).
102 patients have been enrolled, in two arms: 91 patients data records were analyzed:
- 45 patients in the Control group: patients implanted with EVAR only;
- 46 patients in Study group: patients implanted with EVAR and parallel thrombogenic fibers embolization of the aneurysm sac.
With a 24-month follow-up after implant, the Study group of patients has shown a dramatic improvement of the freedom from endoleaks and secondary interventions, and the reduction of the aneurysm volume and diameter.
- No complications related to thrombogenic fibers embolization were observed.
- Rate of secondary operations and endoleaks was significantly reduced in the Study Group (p= 0.003) from 78% to 47%.
- A significant reduction of aneurysm volume of about 55% was observed in patients in the Study Group Vs the Control Group at 24 months (p=0.001).
Professor Dominique Fabre, indicates:
“This is a long-awaited clinical improvement in EVAR outcome that can be standardized in a ready to use thrombogenic fibers coated prosthesis providing the same functional embolization as in the SCOPE 1 study.”
Daniele Zanotti, CEO of AFFLUENT MEDICAL said:
“KARDIOZIS proprietary technology has been conceived to achieve the same results but avoiding the complexity of an additional procedure, by effectively preventing type II endoleaks which is the main complication after an EVAR implant. This trial confirms, with strong evidence, the therapeutic value of the KARDIOZIS technology, which has the potential of achieving far superior clinical outcomes, reduced need for reintervention and fewer control visits.
KARDIOZIS technology, proven in the SCOPE 1 trial, can be applied both to existing endoprosthesis on the market, via corporate partnerships, and to Affluent own endoprosthesis under development. We thank Prof. Fabre and the whole team of investigators which has conducted the SCOPE 1 trial to this spectacular result.”
For more information on the KARDIOZIS technology:
- SCOPE 1 Results: Professor Fabre presentation at the CACVS in Paris
KARDIOZIS, a promising implant to be launched by 2021 in Europe
The first endovascular prosthesis to prevent endoleaks in life threatening abdominal aortic aneurysm, which affects 5 to 10% of men from age 65 to 801. Ruptured abdominal aortic aneurysm if mostly fatal.
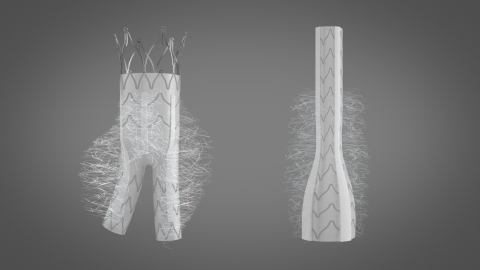
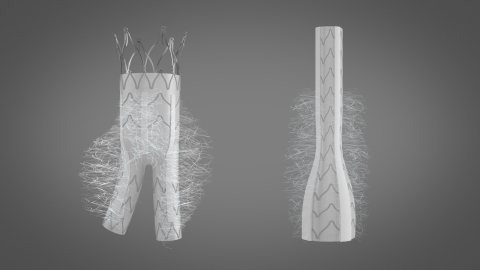
From 20% to 40% of cases, type II endoleaks (collateral aortic artery reflux) occur after endovascular aneurysm repair with existing stent-grafts and present a severe risk if left untreated. The KARDIOZIS device helps to reduce endoleaks and the size of the aneurysm, and then prevents such complications. KARDIOZIS, currently in preclinical development, aims to become the leading treatment for abdominal aortic aneurysm. Its European launch is planned for 2021, in a global market growing at a rate of 6.6% per year between 2017-2023 and expected to reach $3.6 billion by 20232.
KARDIOZIS is a proprietary minimally invasive ready-to-use prosthesis which differs from the other endovascular prostheses that currently dominate the market as it has been designed with thrombogenic fibers on the outer wall of the prosthesis. These fibers have a length and distribution that allows them to embolize the aneurysm after implantation, as potently as the thrombogenic fibers that were inserted in the aneurysm sac in the SCOPE-1 trial after the implantation of a conventional endovascular prosthesis.
Switch from « Wait & See » to « Treat » attitude. This new technology is expected to stimulate next market growth.
Since its introduction in 1991, EVAR procedures have been increasingly adopted by vascular surgeons worldwide. Physicians are more inclined to treat AAA by percutaneous EVAR and avoid complex open surgery, due to the shorter procedure time and increased efficacy of stent-grafts, as well as to a lower complication rate for reaching the AAA site and a reduced rehabilitation time for the patient.
However, in the current EVAR prostheses, various types of endoleaks, including type II ones, continue to constitute one of the main complications of the EVAR procedure and limit its use.
Lifestyle and demographic factors (age, stress, obesity, smoking etc.) greatly contribute to increase the AAA prevalence and risk. Smoking, for example, is responsible for around 20% of cases of aortic aneurysm3. At the same time, more favorable reimbursement schemes and product technological progress stimulate the growth of the market for therapies. However, lack of awareness, insufficient diagnosis (only 20% of AAAs are diagnosed4), strict regulatory approval procedures and risks associated to the current procedures hinder the growth of the market. Anxiety of patients monitored for AAA size modification and risk of fatal rupture if not treated explain the high medical need.
About Affluent Medical
Affluent Medical is a French medtech company founded by Truffle Capital with the ambition to become a European leader in the treatment of heart and vascular diseases, which are the world’s leading cause of death, and of urinary incontinence, which today affects one in four adults. Affluent Medical is developing innovative, next-generation minimally invasive implants to restore key physiological functions in these areas. The company’s four major technologies are currently in preclinical and clinical phases, and a first medical device is expected to be launched by 2021.
For more information: www.affluentmedical.com
1 Vardulaki KA, Prevost TC, Walker NM et al. « Incidence
among men of asymptomatic abdominal aortic aneurysms: estimates from 500
screen detected cases » J Med Screen, 1999;6:50-4
2
Infoholic Research – 2017: Global Aortic Aneurysm Market – Drivers,
Opportunities, Trends and Forecasts 2017-2023
3 Source:
Infoholic Research – 2017: Global Aortic Aneurysm Market – Drivers,
Opportunities, Trends and Forecasts 2017-2023
4 Source:
Infoholic Research – 2017: Global Aortic Aneurysm Market – Drivers,
Opportunities, Trends and Forecasts 2017-2023