PARIS--(BUSINESS WIRE)--Regulatory News:
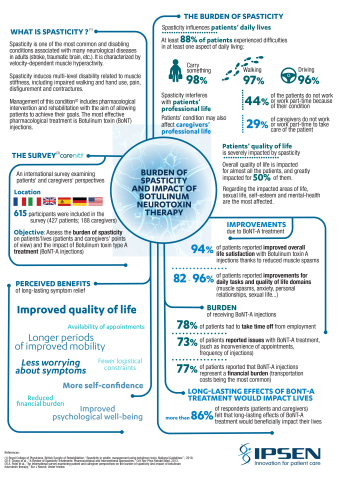
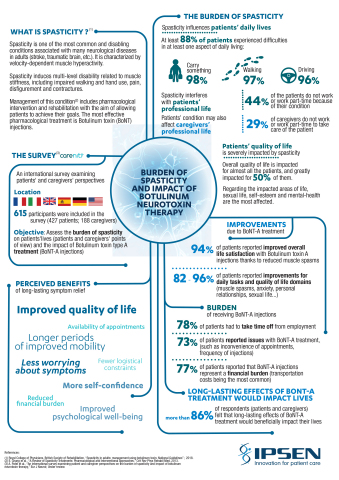
Ipsen (Euronext: IPN; ADR: IPSEY) today presents at TOXINS, results from an international survey revealing the hidden burden of spasticity and the need for longer periods of symptomatic relief1. Affecting 12 million people around the world2, spasticity is one of the most common and disabling conditions associated with neurological diseases in adults (stroke, traumatic brain, etc.) and characterised by an abnormal increase in muscle tone or stiffness3. A survey of 615 respondents from 6 participating countries confirms that spasticity has a profound impact on ability to perform everyday tasks, including the ability to carry items, walk, and drive and reduces independence overall.
The survey1 also found that spasticity affects the ability to work (22% of patients surveyed did not work) and impacts sex life as well as self-esteem. For 94% of patients surveyed, satisfaction with life improves with botulinum toxin type A treatment - injection of BoNT-A is one of the reference treatments for spasticity that acts by blocking neuromuscular transmission4.
Alexandre Lebeaut, M.D., Executive Vice President, Research & Development and Chief Scientific Officer, Ipsen stated: “Spasticity is not always the first symptom that is managed in adult or children central nervous system insult, but it has a long term and chronic profound impact on fundamental aspects of patients and caregivers’ daily lives. The hallmark of good patient care is providing access to effective treatments that can control symptoms, and improve quality of life.”
The survey1, undertaken in association with Carenity, a social media platform for people living with chronic diseases, also exposes the practical issues related to spasticity and its treatment. Most patients (78%) have to take time off work because of their condition. Treatment also represents a financial burden with average out-of-pocket expenses of 150 Euros per injection. 9 out of 10 respondents want long periods without symptoms, and expect it would have a positive impact on their quality of life. An interim analysis of ULIS-III5 – a phase IV study on attainment of person-centered goals after BoNT-A treatment for adult upper limb spasticity – reporting on treatment intervals is presented at TOXINS 2019.
Jorge Jacinto, PM&R Senior Consultant, Head of Department of Adult Neuro-rehabilitation, Centro de Medicina de Reabilitação de Alcoitão, Portugal, concluded: “The Carenity survey as well as observational studies like ULIS-III5 provide priceless patients insights to clinicians. It will allow us to not only consider the burden of spasticity in its entirety, but also rethink the treatment paradigm to improve patients’ and caregivers’ quality of life.”
Ipsen will be presenting the results of this survey as part of 50 posters submitted to TOXINS 2019 in Copenhagen, which include:
- Burden of spasticity among patients and caregivers: results of a multinational survey; Patel et al.
- The patients’ perspective on botulinum neurotoxin A treatment: results of a multinational survey for patients with spasticity; Bahroo et al.
- Fewer injections of botulinum toxin type A for treatment of spasticity are perceived as beneficial by both patients and caregivers; Wein et al.
- Time to retreatment with botulinum toxin A in upper limb spasticity management: upper limb international spasticity (ULIS)-III study interim analysis; Turner-Stokes et al.
About the survey
Individuals (615 respondents: 69% patients and 31% caregivers) from Western Europe and the USA were asked to complete a survey via the online platform Carenity. Eligible participants were over 18 years old and had (or cared for someone with) spasticity treated with BoNT-A for at least one year. To assess burden of spasticity for patients and caregivers, participants were asked about the impact of spasticity (on ability to work, functioning and quality of life) and of BoNT-A therapy (on their lives and potential benefits of fewer injections).
About spasticity
Spasticity is a condition characterised by velocity-dependent muscle hyperactivity3. Spasticity is usually caused by damage to nerve pathways in the brain or spinal cord that control muscle movement, and may occur in association with cerebral palsy, spinal cord injury, multiple sclerosis, stroke, and brain or head trauma 2–4.Spasticity, is experienced by 34% of stroke survivors within one year after a first stroke6–8. Around 84% of patients with multiple sclerosis live with some form of spasticity2.
References:
- Patel, A. et al. An international survey examining patient and caregiver perspectives on the burden of spasticity and impact of botulinum neurotoxin therapy. Eur J Neurol.
- AANS. AANS Website - Spasticity.
- AAN. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache. (2016). doi:10.1212/WNL.0000000000002560
- Dystonia.org.uk. Dystonia explained. 1–4 (2014).
- Turner-Stokes, L. et al. Impact of integrated upper limb spasticity management including botulinum toxin A on patient-centred goal attainment: rationale and protocol for an international prospective, longitudinal cohort study (ULIS-III). Open 6, 11157 (2016).
- Kuo, C. L. & Hu, G. C. Post-stroke Spasticity: A Review of Epidemiology, Pathophysiology, and Treatments. Int. J. Gerontol. 1–5 (2018). doi:10.1016/j.ijge.2018.05.005
- Thibaut, A. et al. Spasticity after stroke: Physiology, assessment and treatment. Brain Inj. 27, 1093–1105 (2013).
- Esquenazi, A. The human and economic burden of poststroke spasticity and muscle overactivity. J. Clin. Outcomes Manag. 18, 34–44 (2011).
About Ipsen
Ipsen is a global biopharmaceutical group focused on innovation and specialty care. The group develops and commercializes innovative medicines in three key therapeutic areas - Oncology, Neuroscience and Rare Diseases. Its commitment to Oncology is exemplified through its growing portfolio of key therapies for prostate cancer, neuroendocrine tumors, renal cell carcinoma and pancreatic cancer. Ipsen also has a well-established Consumer Healthcare business. With total sales over €1.9 billion in 2017, Ipsen sells more than 20 drugs in over 115 countries, with a direct commercial presence in more than 30 countries. Ipsen's R&D is focused on its innovative and differentiated technological platforms located in the heart of the leading biotechnological and life sciences hubs (Paris-Saclay, France; Oxford, UK; Cambridge, US). The Group has about 5,400 employees worldwide. Ipsen is listed in Paris (Euronext: IPN) and in the United States through a Sponsored Level I American Depositary Receipt program (ADR: IPSEY). For more information on Ipsen, visit www.ipsen.com.
Forward Looking Statement
The forward-looking statements, objectives and targets contained herein are based on the Group’s management strategy, current views and assumptions. Such statements involve known and unknown risks and uncertainties that may cause actual results, performance or events to differ materially from those anticipated herein. All of the above risks could affect the Group’s future ability to achieve its financial targets, which were set assuming reasonable macroeconomic conditions based on the information available today. Use of the words "believes", "anticipates" and "expects" and similar expressions are intended to identify forward-looking statements, including the Group’s expectations regarding future events, including regulatory filings and determinations. Moreover, the targets described in this document were prepared without taking into account external growth assumptions and potential future acquisitions, which may alter these parameters. These objectives are based on data and assumptions regarded as reasonable by the Group. These targets depend on conditions or facts likely to happen in the future, and not exclusively on historical data. Actual results may depart significantly from these targets given the occurrence of certain risks and uncertainties, notably the fact that a promising product in early development phase or clinical trial may end up never being launched on the market or reaching its commercial targets, notably for regulatory or competition reasons. The Group must face or might face competition from generic products that might translate into a loss of market share. Furthermore, the Research and Development process involves several stages each of which involves the substantial risk that the Group may fail to achieve its objectives and be forced to abandon its efforts with regards to a product in which it has invested significant sums. Therefore, the Group cannot be certain that favorable results obtained during pre-clinical trials will be confirmed subsequently during clinical trials, or that the results of clinical trials will be sufficient to demonstrate the safe and effective nature of the product concerned. There can be no guarantees a product will receive the necessary regulatory approvals or that the product will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements. Other risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the Group's ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the Group’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions. The Group also depends on third parties to develop and market some of its products which could potentially generate substantial royalties; these partners could behave in such ways which could cause damage to the Group’s activities and financial results. The Group cannot be certain that its partners will fulfil their obligations. It might be unable to obtain any benefit from those agreements. A default by any of the Group’s partners could generate lower revenues than expected. Such situations could have a negative impact on the Group’s business, financial position or performance. The Group expressly disclaims any obligation or undertaking to update or revise any forward looking statements, targets or estimates contained in this press release to reflect any change in events, conditions, assumptions or circumstances on which any such statements are based, unless so required by applicable law. The Group’s business is subject to the risk factors outlined in its registration documents filed with the French Autorité des Marchés Financiers. The risks and uncertainties set out are not exhaustive and the reader is advised to refer to the Group’s 2017 Registration Document available on its website (www.ipsen.com).