CAMBRIDGE, Mass.--(BUSINESS WIRE)--Myomo, Inc., a wearable medical robotics company, has achieved recent momentum fitting and delivering MyoPro to adolescent users. Approved to assist young adults suffering from any disease or injury leaving them with a paralyzed or weakened arm, MyoPro is restoring motion and functionality to adolescents with conditions such as cerebral palsy, brachial plexus injury, and more. MyoPro is now being tested on Acute Flaccid Myelitis ("AFM") patients with promising results.
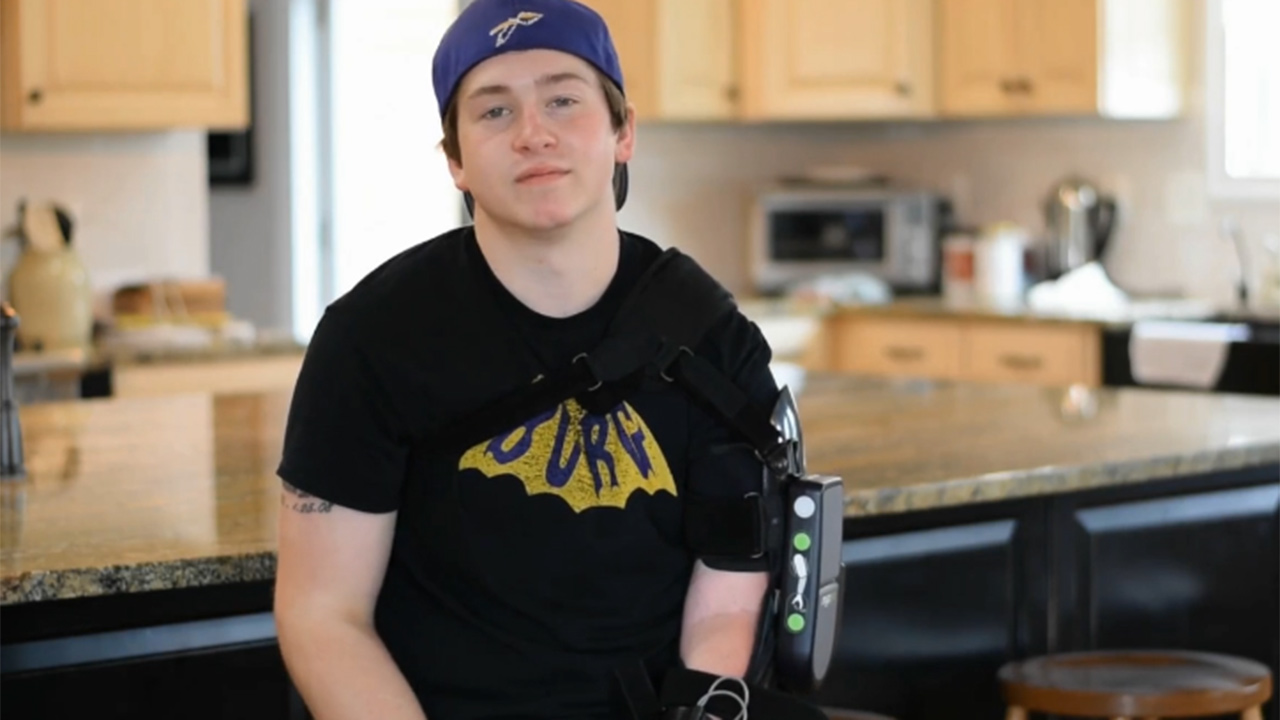
The first adolescent user to be fitted, Zeke Dees, has regained his independence after almost a decade of only having use of one arm and credits his MyoPro device for transforming his life. When he was 8 years old, he suffered a life-altering ATV accident. At the hospital, doctors discovered that he suffered a brain injury, a spinal cord injury, and a brachial plexus injury. Zeke received surgery and significant, comprehensive therapy and made a remarkable recovery, except for the brachial plexus injury (BPI) to his shoulder, leaving his arm completely paralyzed. Subsequently, Zeke had to try to do everything again with one functional arm.
Use of the MyoPro robotic brace is continuing to expand and is reaching youth through highly regarded rehabilitation centers including Easterseals, Shriner’s Hospitals and esteemed children’s hospitals in several cities including Cleveland, Boston, Dallas and Cincinnati. MyoPro has more recently been fitted and delivered to cerebral palsy patients at some of these facilities with promising early results.
“The brachial plexus is the network of nerves that sends signals from your spinal cord to your shoulder, arm, and hand. There aren’t a lot of non-invasive, clinical interventions for BPI that produce the same results as the MyoPro,” said Liz May, pediatric occupational therapist at Shriners Hospital for Children in Chicago. “With the help of the MyoPro, we are seeing patients drastically improve their range of motion, spontaneous use, and function of their involved upper extremity, despite paralysis. This device has allowed my patients to find a new state of independence, which has completely transformed their outlook on life.”
After his injury, Zeke tried to establish a new sense of normalcy and considered the reality of spending the rest of his life only having one functioning arm. Since being fitted for a MyoPro, Zeke has completely changed his attitude and has been able to resume activities his injury previously hindered him from doing such as cutting his food, playing pool and helping with chores around the house.
“My MyoPro device has changed my life dramatically. Not only has it allowed me to gain my independence back, but it’s brought strength back to my left arm,” said Zeke Dees, an adolescent Myomo user. “I got my brace when I was 17 and I can’t imagine life without it. It’s already helped me with the activities of daily living that I couldn’t do before.”
Paul R. Gudonis, Chairman and CEO of Myomo, said, “Zeke is just one of many success stories from Myomo patients who have significantly regained the use of a once paralyzed limb. His personal story is yet another example of Myomo’s commitment to helping restore function in the arms and hands of individuals who are suffering from the effects of a neuromuscular disease or injury. We are looking forward to providing more adolescents a chance to improve their quality of life, just like Zeke has.”
Through patented technology, the MyoPro orthosis can offer individuals living with a paralyzed arm the opportunity to regain function, increasing safety and quality of life in the home and reducing healthcare costs associated with managing upper limb paralysis.
For more information about MyoPro for adolescents, please visit www.myomo.com/adolescents.
About Myomo
Myomo, Inc. is a wearable medical robotics company that offers expanded mobility for those suffering from neurological disorders and upper limb paralysis. Myomo develops and markets the MyoPro product line. MyoPro is a powered upper limb orthosis designed to support the arm and restore function to the weakened or paralyzed arms of patients suffering from CVA stroke, brachial plexus injury, traumatic brain or spinal cord injury, ALS or other neuromuscular disease or injury. It is currently the only marketed device that, sensing a patient’s own EMG signals through non-invasive sensors on the arm, can restore an individual’s ability to perform activities of daily living, including feeding themselves, carrying objects and doing household tasks. Many are able to return to work, live independently and reduce their cost of care. Myomo is headquartered in Cambridge, Massachusetts, with sales and clinical professionals across the U.S. For more information, please visit www.myomo.com.
Forward Looking Statements
This press release contains forward-looking statements regarding the Company's future business expectations, including adoption of MyoPro by adolescent users, which are subject to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are only predictions and may differ materially from actual results due to a variety of factors. Our actual results could differ materially from those anticipated in these forward looking statements for many reasons, including, without limitation, risks related to regulatory approval and market acceptance of our products, and the other risk factors contained in our filings made with the Securities and Exchange Commission. More information about factors that potentially could affect Myomo's business and financial results is included in Myomo's filings with the Securities and Exchange Commission. The Company cautions readers not to place undue reliance on any such forward-looking statements, which speak only as of the date made. The Company disclaims any obligation subsequently to revise any forward-looking statements to reflect events or circumstances after the date of such statements or to reflect the occurrence of anticipated or unanticipated events.