TOKYO--(BUSINESS WIRE)--Augmenix K.K. announces that SpaceOAR hydrogel, a soft, implanted absorbable gel spacer is now available to all prostate cancer radiotherapy patients in Japan through the Ministry of Health, Labour & Welfare (MHLW) national reimbursement. SpaceOAR hydrogel is clinically proven to assist in the decrease of unwanted side effects from radiotherapy treatment in patients with prostate cancer. 1,2
Stephen J. McGill, Vice President and General Manager of Augmenix International stated, “We are delighted that SpaceOAR hydrogel is now available through the MHLW national reimbursement programme for all men undergoing prostate cancer radiotherapy treatment throughout Japan. We believe the commitment of the Japanese MHLW to reimburse SpaceOAR is a significant leap forward to improving radiotherapy outcomes and reducing the associated risks.”
Stephen McGill continued to say, “It is our goal to be able to provide global access to SpaceOAR hydrogel, a minimally invasive intervention, that significantly benefits patients by reducing the side effects of radiation exposure. The opening of our new Augmenix K.K. headquarters and successful reimbursement are positive steps in that direction.”
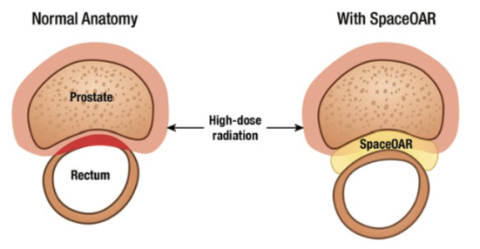
Throughout Japan, more than 86,000 men are diagnosed with prostate cancer every year. Many patients are successfully treated with various forms of radiotherapy, however radiation damage to the organs surrounding the prostate are known to cause long term side effects including acute rectal pain, rectal bleeding, urinary incontinence and erectile dysfunction. SpaceOAR hydrogel creates an average of 1.3cm of space between the prostate and the rectum which has been clinically proven to reduce radiation exposure and prevent injury to the surrounding organs.1,2
Yoshi Kuratani, General Manager of Japan, continued to emphasise, “SpaceOAR hydrogel is a proven solution that can make a life-changing difference to men receiving prostate cancer radiotherapy. We hope that by working closely with the many Japanese clinicians that have already expressed an interest in this product, we can help enable the advancement of radiotherapy and make prostate-rectum spacing the standard of care for all patients.”
SpaceOAR hydrogel is available nationally in Japan beginning June 25th, 2018 under the Reimbursement Category: ‘200 Bio absorbable material for Radiation Therapy’. More information is available at http://www.spaceoar.com
References
1. Hamstra D, et al. Continued Benefit to Rectal Separation for Prostate
RT: Final Results of a Phase III Trial. Int J Radiat Oncol Biol Phys;
2017 Volume 97, Issue 5, Pages 976–985.
2. Hamstra D, et al.
Evaluation of sexual function on a randomized trial of a prostate rectal
spacer. J Clin Oncol 35, 2017
Notes to Editor
About Augmenix: Augmenix, Inc. is a company focused on the development and commercialisation of radiation oncology products using its proprietary hydrogel technology. Focusing initially on protection during prostate radiation therapy, Augmenix next-generation products will address spacing and marking applications throughout the body to improve radiotherapy and interventional oncology procedure outcomes. The company’s lead product, SpaceOAR hydrogel, is FDA cleared and it is currently being used in many leading cancer centres in the United States, Australia and Europe. It is CE marked, Shonin approved for Japan, approved in Australia and licensed in Canada. SpaceOAR is a registered trademark of Augmenix, Inc. More information about Augmenix and the SpaceOAR Hydrogel can be found at http://www.spaceoar.com.
About SpaceOAR Hydrogel: In a prospective, randomized, multi-center clinical trial in the United States, patients treated with SpaceOAR hydrogel prior to prostate cancer radiation treatment demonstrated bowel, urinary, and sexual benefits through three years median of follow-up. The study found that the patients who did not receive SpaceOAR hydrogel experienced a clinically significant decline in bowel, urinary, and sexual quality of life eight times more often than patients who received SpaceOAR hydrogel. (1,2)
SpaceOAR is intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and in creating this space it is the intent of SpaceOAR hydrogel to reduce the radiation dose delivered to the anterior rectum. The SpaceOAR hydrogel is composed of biodegradable material and maintains space for the entire course of prostate radiotherapy treatment. It is completely absorbed by the patient’s body over time. Clinical data comparing patients with and without SpaceOAR hydrogel demonstrated the benefits of SpaceOAR hydrogel to include reduction of rectal toxicity resulting in improved bowel function, improvements in urinary function, and a higher likelihood to maintain sexual function. Potential complications associated with SpaceOAR hydrogel include but are not limited to pain associated with SpaceOAR hydrogel injection; pain or discomfort associated with SpaceOAR hydrogel, needle penetration of the bladder, prostate, rectal wall, rectum, or urethra; injection of SpaceOAR hydrogel into the bladder, prostate, rectal wall, rectum, or urethra; local inflammatory reactions; infection; injection of air, fluid or SpaceOAR hydrogel intravascularly; urinary retention; rectal mucosal damage, ulcers, necrosis; bleeding; constipation; and rectal urgency.