CAMBRIDGE, Mass.--(BUSINESS WIRE)--Genosco, a clinical-stage biotechnology company focused in immunology and oncology, announced data from a Phase 1/2 study evaluating lazertinib (YH25448, GNS-1480) in patients with advanced Non-Small Cell Lung Cancer (NSCLC) at the 2018 American Society of Clinical Oncology (ASCO) Annual Meeting. Lazertinib (YH25448, GNS-1480), Genosco’s 3rd-generation EGFR-tyrosine kinase inhibitor (EGFR-TKI) candidate partnered for clinical development and commercialization with Yuhan Corporation, is an oral, potent, irreversible EGFR-TKI that is highly selective for activating (EGFRm) and T790M resistance mutations.
Results from the open-label, multi-center dose-escalation, Phase 1/2 study of lazertinib (YH25448, GNS-1480) for patients with advanced EGFR-TKI-resistant NSCLC with or without CNS metastasis concluded that lazertinib was well-tolerated with low rates of Grade 3 or higher adverse events (AE) and exhibited robust activity in patients with NSCLC with acquired resistance to EGFR-TKIs, with or without brain metastasis. Principal Investigator Byoung Chul Cho, M.D., Ph.D., Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Republic of Korea presented the data in a poster session (Abstract 9033).
“These data are impressive and underscore the potential of lazertinib (YH25448, GNS-1480) to be the best-in-class 3rd-generation EGFR-TKI for patients with advanced EGFR T790M mutant NSCLC, including brain metastasis. And importantly, the treatment was well-tolerated with no dose-limiting toxic effects,” said Dr. Byoung Chul Cho. “Results indicate that lazertinib compares favorably with results from a similar Phase 1/2 clinical trial of osimertinib (AURA)1, a currently marketed 3rd generation EGFR-TKI.”
“The efficacy signals and safety profiles are highly encouraging and validate lazertinib (YH25448, GNS-1480) as a promising 3rd-generation EGFR-TKI inhibitor for patients with limited options,” said Jong Sung (John) Koh, Ph.D., Genosco CEO. “Yuhan and Genosco initiated a global Phase 2 trial evaluating lazertinib for patients with NSCLC and anticipate a global Phase 3 trial in 2019.”
“These results confirm our belief that recently presented pre-clinical data at AACR2 may translate into human studies,” said Ho-Juhn Song, Ph.D., Director of Biology and Strategic Alliance of Genosco. “The comparative analyses of lazertinib and osimertinib concluded that lazertinib showed greater potency and selectivity, excellent intracranial penetration, superior in vivo efficacy in both single (del19, L858R) and double (L858R/T790M) mutant xenograft models and superior in vivo efficacy in a brain metastasis model.”
Study results:
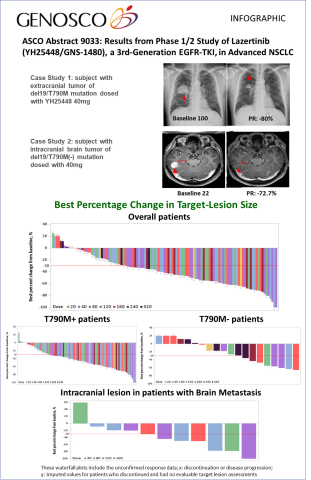
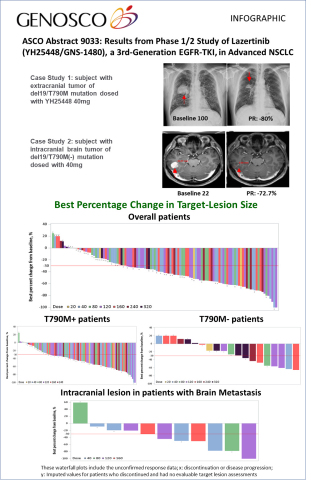
A total of 118 patients [dose-escalation cohort (n=38) and expansion cohort (n=80)] with EGFRm advanced NSCLC with acquired resistance to EGFR-TKIs with or without brain metastasis were enrolled in the Phase 1/2 study as of April 20, 2018.
The results demonstrate that lazertinib (YH25448, GNS-1480) has a good safety profile and was generally well-tolerated. No dose-limiting toxicities were observed up to lazertinib 320mg and there were no dose-dependent increases in treatment-emergent adverse events (TEAEs). Of the evaluable patients (n=110) with a confirmed response at the date of data cutoff, lazertinib (YH25448, GNS-1480) demonstrated promising anti-tumor efficacy signals with a confirmed objective response rate (ORR) of 61% across all dose levels. Of note, the confirmed ORR in patients with T790M+ was 86% at the lazertinib 240mg dose level and in patients with brain metastasis, the intracranial ORR was 55% across all dose levels.
Key findings include:
| Anti-tumor efficacy – all confirmed responses | ||||
| Overall Objective Response Rate (ORR) – all doses | (n=110) | 61% | ||
| ORR - T790M-positive patients – all doses | (n=92) | 66% | ||
| ORR - T790M-positive patients – 240 mg dose | (n=7) | 86% | ||
| ORR - T790M-negative patients – all doses | (n=18) | 33% | ||
| ORR – patients with brain metastasis | (n=11) | 55% | ||
| Tolerability (Adverse Event) | ||||
| Any AEs of grade 3-5 | 11% | |||
| Any drug-related AEs grade 3-5 | 2% | |||
EDITORS NOTE: An infographic accompanying this release is available.
Sources:
1 N
Engl J Med 372;18 nejm.org April 30, 2015; AZD9291 in EGFR
Inhibitor–Resistant Non–Small-Cell Lung Cancer.
2
AACR
2018 Annual Meeting Abstract Number 4790: YH25448, an irreversible
3rd-generation EGFR TKI, exhibits superior anticancer effects with
potent brain BBB penetration in NSCLC.
About Lazertinib
Lazertinib (YH25448, GNS-1480) is an oral, potent, highly mutant-selective and irreversible, investigational 3rd-generation EGFR-TKI that penetrates the blood-brain barrier (BBB). It targets the activating EGFR mutations Del19 and L858R, as well as the T790M mutation, while sparing wild type. EGFR mutations are present in approximately 10-15% of NSCLCs. Lazertinib is being evaluated in advanced NSCLC as both first- and second-line treatments.
About Genosco
Located in Cambridge, MA, Genosco is a clinical-stage biotechnology company led by CEO, Dr. Jong Sung (John) Koh, focusing on developing and commercializing novel immunology and oncology treatments to improve the lives of patients. Genosco’s core competence comes from its expertise in generating selective kinase inhibitors that include candidates that target EGFRm+ (NSCLC), SYK (Autoimmune diseases) and FLT3/AXL (AML) and other conditions. The company is enrolling patients in a Phase 1 study of SKI-G-801 for AML and expects to initiate a Phase 2 study of SKI-O-703 in ITP and RA in 2018. In 2015, Genosco partnered globally with Yuhan Corporation for development and commercialization of lazertinib (YH25448, GNS-1480), a 3rd-generation EGFR-TKI, for treatment of NSCLC currently in Phase 2 clinical trials. Genosco was founded in 2008 as a subsidiary company of Oscotec, Inc, (KOSDAQ: 039200), South Korea. For more information, please visit www.genosco.com.
About Yuhan Corporation
Yuhan Corporation is a globally operating, leading pharmaceutical company in South Korea led by CEO Jung Hee Lee. Founded in 1926 by Dr. Ilhan New, the company is engaged in research and development (R&D), drug manufacturing, CMO business, marketing and distribution of a broad spectrum of pharmaceutical and healthcare products. R&D strategies of Yuhan Corporation revolve around, first and the foremost, the patient needs. This patient-centered approach has directed the focus of Yuhan R&D on two critical areas of unmet medical needs; oncology and metabolic diseases. For more information, please visit www.yuhan.co.kr.
Source: Genosco