REDMOND, Wash.--(BUSINESS WIRE)--(Product Bulletin) Today Physio-Control, now part of Stryker, announced its newest version of the LUCAS® 3 Chest Compression System, version 3.1. The latest version provides powerful new capabilities for tailored device functionality, wireless reporting and device status notifications sent over email.
The latest LUCAS 3.1 version allows professional users to tailor setup options for compression rate/depth, pauses, alerts, timer, and ventilation features to meet local emergency care protocols. LUCAS 3.1 now includes Wi-Fi® connectivity with a LIFENET® System account to enable users to set up device readiness notifications, modify setup options and transmit device reports wirelessly to users over e-mail when not in clinical use. Users can set automatic e-mail reports to facilitate post-event reviews and device management reports on battery expiration dates, last service date, and device maintenance.
“LUCAS continues to lead by customer-focused innovation. With this latest version 3.1 of LUCAS, EMS and hospital response teams have more flexibility to support their local care protocols and can concentrate on making real-time, critical decisions during a cardiac event,” says Erik von Schenck, VP Research and Development, Physio-Control. “The advanced capabilities with wireless transmission enables our customers to do more with their LUCAS device and proactively manage assets, device maintenance and post-event reviews.”
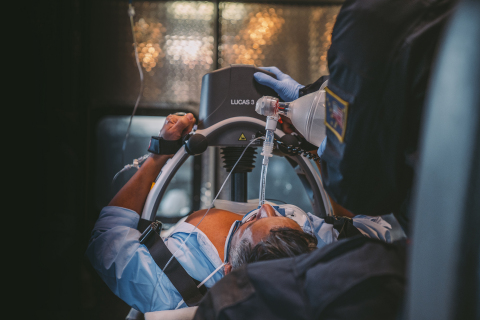
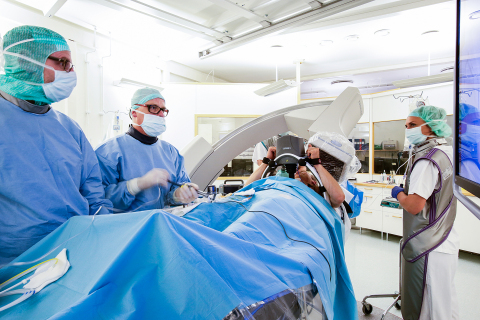
For over 15 years, the LUCAS Chest Compression System has been helping lifesaving teams around the world deliver high-quality, chest compressions for adults experiencing sudden cardiac arrest. LUCAS is an external mechanical device that delivers uninterrupted automatic chest compressions at a rate and depth that are consistent with current American Heart Association (AHA) and European Resuscitation Council (ERC) guidelines for cardiopulmonary resuscitation (CPR).
LUCAS helps provide high-quality compressions in situations where manual CPR may be dangerous or challenging for providers, such as when performing CPR in a moving ambulance. The device enhances medical providers’ safety by enabling rescuers to sit and wear seatbelts during ambulance or helicopter transport, rather than manually providing compressions in a moving vehicle or aircraft.
The LUCAS 3 Chest Compression System, version 3.1 received 510(k) clearance in February 2018. More details on LUCAS are available at physio-control.com/LUCAS.
About Physio-Control, now part of Stryker
Physio-Control, now part of Stryker, is the world’s leading provider of professional emergency medical response solutions that predict or intervene in life-threatening emergencies. The company’s products include LIFEPAK® monitor/defibrillators and automated external defibrillators, LUCAS® Chest Compression Systems, the LIFENET® System, HeartSine® AEDs and more.
Learn more at physio-control.com, or connect on Facebook, LinkedIn or Twitter
Product Specifications
| Brand name | LUCAS® 3 Chest Compression System, version 3.1 | |||
| Basic machine weight | Weight of the device with Battery (no straps): 17.7 lbs / 8.0 kg | |||
| Dimensions | Device dimensions when assembled (HxWxD): 22.0 x 20.5 x 9.4 inches / 56 x 52 x 24 cm | |||
| Operation | Battery run time (nominal patient): Battery run time 45 minutes (typical) Extended run time connecting to external power supply | |||
| Communications | Wireless connectivity: Device can communicate via Bluetooth™ and connect to configured Wi-Fi networks to receive and transmit data when not in clinical use. | |||
| Device and Therapy | Type of chest compression | |||
| • Piston with suction cup designed to stabilize the compression point | ||||
| • Suction cup may assist chest recoil back to the start position | ||||
| • Factory default settings consistent with AHA and ERC Guidelines 2015 | ||||
| Compression rate | ||||
| • Configurable to 102 – 111 – 120 compressions per minute, fixed, or variable during use | ||||
| • Factory default setting: 102 ± 2 compressions per minute |