DANVERS, Mass.--(BUSINESS WIRE)--Please replace the release dated March 23, 2018 with the following corrected version due to multiple revisions.
The corrected release reads:
SAMSUNG RECEIVES FDA CLEARANCE FOR PREMIUM ULTRASOUND SYSTEM RS85
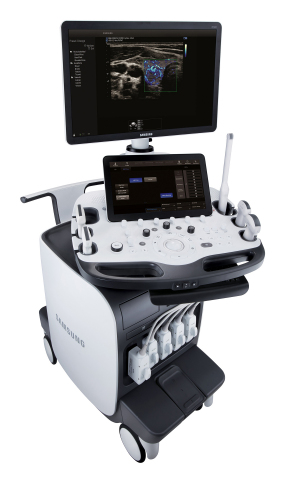
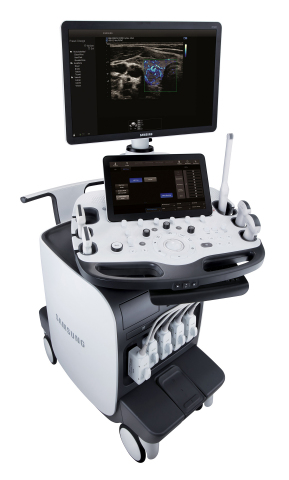
The Samsung RS85 premium ultrasound system combines exceptional image quality with advanced ergonomics
NeuroLogica, a subsidiary of Samsung Electronics Co. Ltd., announced that the Samsung RS85 ultrasound system has received FDA 510(k) clearance. The RS85 is the latest expansion of Samsung’s revolutionary ultrasound portfolio.
“We are pleased to launch the RS85, a new premium general imaging ultrasound system with superior image quality and usability based on Samsung’s advanced ultrasound and radiology technologies,” said Philip Sullivan, President and CEO of NeuroLogica. “The RS85 embodies Samsung’s commitment to providing leading technologies to healthcare providers by combining our key values of access, accuracy and efficiency.”
The release of the RS85 is the latest demonstration of Samsung’s leadership in ultrasound. It features an array of improvements, including:
- MV-Flow™: Allows for visualization of slow-flow micro vascularized structures, which can be difficult to assess with conventional power Doppler ultrasound. It also provides clinicians an additional way to check lesions for indications of cancer or inflammation.
- S-Shearwave Imaging™: Provides new indicators for clinical diagnosis by quantifying the elasticity of tissue or lesions via shearwave elastography, which may help increase the accuracy of diagnosis for breast and liver diseases.
- CEUS+: Diagnoses and characterizes lesions in the liver using a contrast agent in adult and pediatric populations.
- S-Fusion™: Enables simultaneous localization of a lesion using real-time ultrasound in conjunction with other volumetric imaging modalities such as an MRI or a CT, while expanding its capabilities to the prostate gland for precise, targeted biopsy guidance.
The RS85 was also developed to help decrease user-fatigue and repetitive motions with an enhanced monitor arm and increased range of motion and tilt. These improvements allow the user to position the monitor for optimal viewing and control. Additionally, multi-step actions have been combined into a single step to help reduce keystrokes and repetitive user interface interactions.
“Samsung has built innovative and cutting-edge diagnostic ultrasound products for more than 30 years,” said David Legg, Vice President of Sales at NeuroLogica. “We are so confident that our RS85 ultrasound system will perform reliably and be the system that you can depend on for years to come, that we are proud to offer a 5-year warranty standard, at no additional cost.”
Learn more by following NeuroLogica on Facebook, Twitter and LinkedIn.
About NeuroLogica
NeuroLogica,
the healthcare subsidiary of Samsung Electronics Co., Ltd., develops,
manufactures and markets innovative imaging technologies, and is
committed to delivering fast, easy and accurate diagnostic solutions to
healthcare providers. NeuroLogica, the global corporate headquarters and
manufacturer of Samsung computed tomography, is also the US headquarters
for sales, marketing and distribution of all Samsung digital radiography
and ultrasound systems. NeuroLogica’s growing portfolio of advanced
medical technologies are used worldwide in leading healthcare
institutions, helping providers enhance patient care, improve patient
satisfaction and increase workflow efficiency. Samsung is committed to
being leaders in the field of healthcare imaging. For more information,
please visit www.SamsungNeuroLogica.com.
About Samsung Electronics Co., Ltd.
Samsung
inspires the world and shapes the future with transformative ideas and
technologies. The company is redefining the worlds of TVs, smartphones,
wearable devices, tablets, digital appliances, network systems and
memory, system LSI, foundry and LED solutions. For the latest news,
please visit the Samsung Newsroom at http://news.samsung.com/.