TOKYO--(BUSINESS WIRE)--Morinaga Milk Industry Co., Ltd. (TOKYO:2264), a leading Japanese dairy product company, today announced the results of a new study investigating the preventive effects of its new probiotic strain Bifidobacterium breve A1 on a model of Alzheimer’s disease. Researchers found that B. breve A1 improved spatial recognition capability, as well as learning and memory capabilities, in cognitively deficient mice, indicating it could play an important role in preventing the onset of Alzheimer’s Disease in humans.1
The number of patients affected by dementia is increasing worldwide. One report estimates there were 46.8 million people worldwide living with dementia in 2015 and projects this number will reach 131.5 million by 2050.2 Alzheimer’s disease accounts for a large proportion of dementia cases. Like many chronic diseases, Alzheimer’s develops slowly for several decades before the onset of symptoms, even though deterioration in the brain begins in the early stages. Once the disease has developed, it is difficult to reverse it or even halt its progression; therefore, finding effective countermeasures to prevent the disease’s onset is a top priority for researchers.
The “gut‒brain axis,” which is the functional linkage between the brain and gut microbiota, has attracted attention worldwide. Because probiotics are known to have beneficial effects on gut microbiota, they are a promising treatment for brain health. Indeed, both bifidobacteria and lactobacillus have shown beneficial effects on anxiety and depression.
Building on this research, Morinaga Milk investigated the ability of probiotics to prevent the progression of Alzheimer’s disease in collaboration with Professor Keiko Abe from The University of Tokyo and Kanagawa Institute of Industrial Science and Technology. The study, published in the journal Scientific Reports, revealed the potential of B. breve A1 to prevent the onset of Alzheimer’s disease.
Study Method
Mice were injected with amyloid β — the substance presumed to cause Alzheimer’s disease — in order to induce cognitive dysfunction in a model of Alzheimer’s disease. Mice in the active treatment group were administered B. breve A1 (1 × 109 per day) orally for 10 days (B. breve A1 group). Mice in the control group were given either saline (saline group) or a cholinesterase inhibitor, a medication prescribed for dementia (positive control group). Cognitive function was evaluated by a Y maze test and a passive avoidance test.
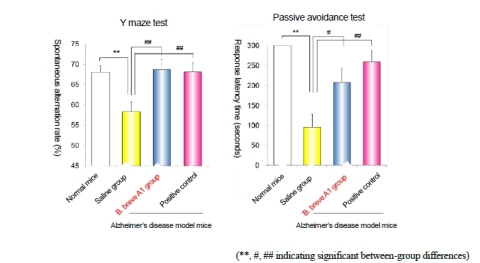
Y maze Behavioral Test Confirms Improvement of Spatial Recognition Capability
“Spontaneous alternation” refers to the tendency of normal mice to choose a different path when navigating a maze than the last one chosen, indicating they remember what paths they have already tried. In this study, amyloid β injected mice given saline showed a remarkable reduction in spontaneous alternation rate in the Y maze test compared to normal mice. However, the B. breve A1 group showed a significant improvement in spontaneous alternation rate compared to the saline group. These results indicate that B. breve A1 improves spatial recognition capability (Fig. 1, left).
Passive Avoidance Behavioral Test Reveals Improvement in Learning and Memory Capability
During the passive avoidance test, mice need to learn to avoid a negative stimulus by staying away from a certain area. If they do so successfully, it indicates they remember the negative experience. Similar to the Y maze behavioral test, mice in the B. breve A1 group stayed longer in the area without a negative stimulus compared to the saline group. This result indicates that B. breve A1 improves learning and memory capabilities (Fig. 1, right).
B. breve A1 Nearly as Effective as Common Alzheimer’s Drug
The improvements in both the Y maze test and the passive avoidance test were similar in magnitude to those observed in the positive control group treated with a cholinesterase inhibitor, a medication commonly prescribed for Alzheimer’s disease, indicating that B. breve A1 has similar potential to improve amyloid-induced cognitive impairment.
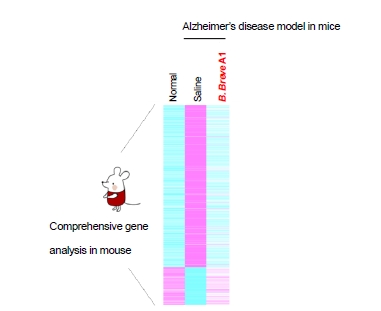
Hippocampus Gene Expression is Key Behind Mechanism of Action
Morinaga Milk focused on the hippocampus, the area of the brain associated with memory and learning capabilities, by performing comprehensive gene expression analysis in the hippocampus of the mice. The researchers found the expression of many genes was altered; particularly, gene groups associated with immunological reactions and inflammation were changed, causing immunological abnormalities and excessive inflammation in the impaired mice ingesting saline. In contrast, the expression of the majority of these genes remained normal in impaired mice ingesting B. breve A1 (Fig. 2).
These findings led Dr. Jin-zhong Xiao, General Manager of Morinaga Milk’s Next Generation Science Institute, to state that “Intake of B. breve A1 suppressed excessive immunological reactions and inflammation in the brains of the mice and improved cognitive function.” He went on to add, “Chronic inflammation of the brain is characteristic of Alzheimer’s disease. B. breve A1’s ability to suppress inflammation in the hippocampus is the key behind its ability to prevent progression of the disease.” The company plans to continue to research the effects of B. breve A1 on human subjects to further explore its therapeutic potential for preventing Alzheimer’s disease onset.
About Morinaga Milk
Morinaga Milk Industry Co., Ltd. is one of the leading dairy product companies in Japan. Morinaga Milk started research on bifidobacteria in the 1960s, inspired by the fact that bifidobacteria are the predominant bacteria residing in the intestines of breast-fed infants. In 1969, Morinaga Milk isolated its flagship strain Bifidobacterium longum BB536 from an infant. Morinaga Milk excels in innovative technology and offers various dairy products and other beneficial functional ingredients to customers around the world.
|
Reference |
||
| 1. | Y. Kobayashi et al. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Scientific Reports 7(1). Dec. 2017. | |
| 2. | ||