TOKYO--(BUSINESS WIRE)--Ajinomoto Co., Inc. (TOKYO: 2802) announces this newsletter:
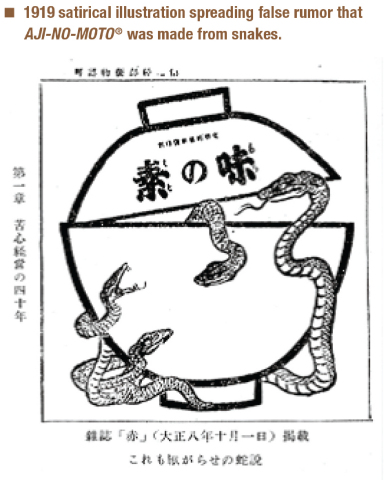
Made From Snakes?
Ajinomoto Co., Inc. (“Ajinomoto Co.”) has been in business for more than 100 years, and that’s a pretty long time. Back in 1908, when Professor Kikunae Ikeda of Tokyo Imperial University first patented the manufacturing process of monosodium glutamate (MSG) from flour, a long-distance radio message was sent from the Eiffel Tower for the first time. New York City passed a regulation making it illegal for women to smoke in public. The United States added a 46th star to its flag for Oklahoma. Mother’s Day was observed for the first time. Henry Ford’s company built its first Model T car, and Orville Wright made the first hour-long airplane flight1.
The world has changed a lot over the past 100 years, but some things always seem to remain the same. For example: it’s really hard to stop a baseless rumor.
Less than ten years after AJI-NO-MOTO®, umami seasoning began sales in Japan, Ajinomoto Co. faced its first public relations crisis. Somehow, somewhere, somebody started a terrible rumor about the product—that it was made from snakes. Where did this idea come from? Nobody knows. But, like rumors tend to do, this idea managed to spread from household to household in Japan.
Of course, AJI-NO-MOTO®, was not, and has never been, made from snakes. At the time, it was made from wheat. But the false rumor presented Ajinomoto Co. with a major challenge. How could they convince the public of the scientific truth? Television advertising wasn’t an option—the first Japanese television wasn’t manufactured over 30 years later2. Radio wasn’t even an option yet. Ajinomoto Co. ran a newspaper advertisement denying the claim9, and even conducted public tastings and hired performers called “chindon-ya” to promote the product’s image in Japan3.
Chinese Restaurant Syndrome
This wasn’t the last time that Ajinomoto Co. had to defend itself against unscientific claims. On April 4, 1968, Dr. H.M. Kwok wrote a Letter to the editor of the prestigious New England Journal of Medicine. He described a “strange syndrome” that he experienced when eating in Chinese restaurants that included a feeling of numbness, weakness, and palpitations, and speculated about several possible causes, including the soy sauce, cooking wine, high sodium content, and—you guessed it—MSG. He concluded by suggesting that one of his colleagues perform a proper scientific investigation into this phenomenon, and offered to help9.
Unfortunately, this completely innocuous Letter to the editor marked the birth of an idea, unsupported by evidence, that MSG caused “Chinese Restaurant Syndrome.” And suddenly, Chinese restaurants everywhere were hanging “No MSG” signs on their front windows—even those that had AJI-NO-MOTO® on the tables inside!
The truth is that after years of research, it’s still not proven whether Chinese Restaurant Syndrome exists at all. But it has been scientifically established that if this syndrome does exist, it definitely is not related to MSG. The final piece of evidence was published by Dr. Geha in 2000, which concluded that added MSG in food does not cause Chinese Restaurant Syndrome9. Nonetheless, decades later, this rumor hasn’t been completely eradicated.
Of Mice and Men
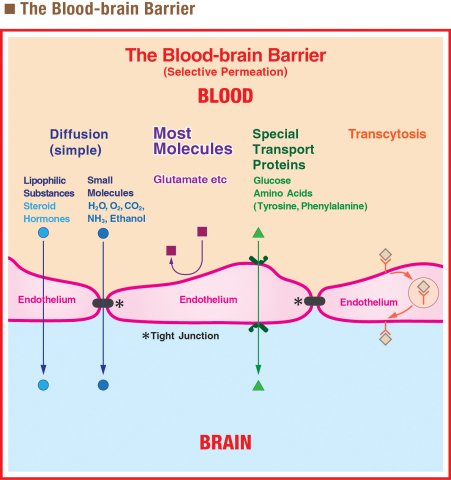
Shortly after Dr. Kwok’s Letter to the editor, in 1969, an alarming study was published in the journal, Science by Dr. J.W. Olney, in which high doses of MSG were injected into newborn mice, which developed brain lesions. However, once again, this turned out to be a false alarm, for two important reasons. First of all, the amount of MSG administered in the study was extremely high—the peroral equivalent range of three bottles (tens to hundreds of grams/per bottle) for an adult-sized subject9. Secondly, and more importantly, there is a major physiological difference between humans and newborn mice that was overlooked in the study.
Mammals have something called the “Blood-brain Barrier” which protects the brain from cells, particles, and specific molecules that are in the bloodstream4. In newborn mice, the Blood-brain Barrier is immature. But primates, including humans, are born with a more mature Blood-brain Barrier. This means that the results observed in mice in this study do not reflect what occurs in humans. And this is why subsequent studies by Dr. Takasaki (1979) and Dr. Helms (2017) have suggested that normal consumption of dietary MSG has no negative effect on the brain9.
Evidence is More Powerful than Rumors
The truth is throughout the years, numerous studies have concluded that MSG is safe. As a result, major regulatory bodies have publicly confirmed this point. The Japanese Ministry of Health, Labour and Welfare, which regulates food safety in Japan, officially approved MSG as a food additive in 19485. Ten years later, the United States Food and Drug Administration recognized MSG as safe6.
In addition, starting in 1970, a Joint Expert Committee on Food Additives (JECFA) formed by the World Health Organization and the Food and Agriculture Organization released a series of statements on the safety of MSG in infants, leading to a 1987 conclusion that there is no need to restrict MSG usage in infants of any age7.
And perhaps the most comprehensive investigation of MSG safety was published in 1995 by the Federation of American Societies for Experimental Biology (FASEB). This report, which addresses 18 detailed questions about MSG safety across more than 350 pages, reaffirms the safety of MSG for the general population at normally consumed levels, finding no evidence connecting MSG to any serious, long-term medical problems8.
Commonly Accepted Scientific Rationale for MSG Safety9
-
Blood glutamate level does not rise when monosodium glutamate is used
with food.
- Approximately 95% of glutamate is metabolized in the intestine for energy.
- Glutamate is the dominant amino acid in breast milk.
- Infants metabolize glutamate as well as adults do, and consume more glutamate than adults relative to body weight without any harmful effect.
- There is no evidence of MSG-related Chinese Restaurant Syndrome.
- Glutamate is a basic taste substance, with its own taste receptors on the tongue.
- MSG intake is “self-limiting”—as with salt or vinegar, using too much actually decreases the palatability of food.
Is There Evidence MSG Might Be Good For People?
Well, for some people, the answer may be “yes.” MSG can be used to increase palatability for people required to consume a salt-restricted diet. For the elderly, as well as for people with nutritional problems, MSG helps counteract loss of appetite9. We will explore these points in detail in future editions of this newsletter.
Ajinomoto Co.—Science is on Our Side
If there’s a bright side to the history of rumors and false claims against MSG, it’s that Ajinomoto Co. has repeatedly responded to these situations with science and evidence. MSG is likely one of the most studied food additive substances in the history of the world. And the continual research Ajinomoto Co. has conducted on its products has made us one of the foremost expert companies on glutamate and other amino acids in the world, which led to our diversification into the fields of science and health.
Ajinomoto Co. will always remain committed to providing not only products that help people eat well and live well, but also the evidence to back these products up.
About Ajinomoto Co., Inc.
Ajinomoto Co. is a global manufacturer of high-quality seasonings, processed foods, beverages, amino acids, pharmaceuticals and specialty chemicals. For many decades Ajinomoto Co. has contributed to food culture and human health through wide-ranging application of amino acid technologies. Today, the company is becoming increasingly involved with solutions for improved food resources, human health and global sustainability. Founded in 1909 and now operating in 30 countries and regions, Ajinomoto Co. had net sales of JPY 1,091.1 billion (USD 10.07 billion) in fiscal 2016. For more about Ajinomoto Co. (TOKYO : 2802), visit www.ajinomoto.com.
For further information or references and literature support of any information contained in this newsletter, please contact Ajinomoto Co., Inc. Global Communications Department: ajigcd_newsletter@ajinomoto.com.
| References: | ||
| 1. |
“Historical Events in 1908,” On This Day, |
|
| 2. |
“1960-1969 Japanese Television Sets,” Television History - The
First 75 Years, |
|
| 3. | Stephanie Assmann, Eric C. Rath (eds.), “Japanese Foodways, Past and Present,” University of Illinois Press, 2010, 152. | |
| 4. |
Oxford, “Oxford Dictionaries,” Oxford University Press, 2017. |
|
| 5. |
“The Ordinance for Enforcement of the Food Sanitation Act,” The
Ministry of Health and Welfare, No.23, July 13, 1948. |
|
| 6. |
“CFR - Code of Federal Regulations Title 21, ” U.S. Food and Drug
Administration, April 1, 2017. |
|
| 7. |
“MONOSODIUM L-GLUTAMATE,” Evaluations of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA), World Health Organization,
1987. |
|
| 8. |
Monica Singh, “FACT OR FICTION? The MSG Controversy,” LEDA at H
arvard Law School, 2005. |
|
| 9. |
Data on file. |
|