PARIS--(BUSINESS WIRE)--Regulatory News:
GenSight Biologics (Paris: SIGHT) (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on discovering and developing innovative gene therapies for neurodegenerative retinal diseases and diseases of the central nervous system, today reported 2.5 years of follow-up data from its Phase I/II clinical trial with the Company’s gene therapy GS010 in patients with Leber Hereditary Optic Neuropathy (LHON). These results confirm long-term sustained gains in visual acuity 2.5 years after a single intravitreal injection of GS010, especially in subjects with less than 2 years of onset of vision loss.
In the study, five cohorts of three subjects were administered an increasing dose of GS010 via a single intravitreal injection in the eye more severely affected by the disease. Recruitment of 15 subjects was completed in April 2015 and long-term follow-up is ongoing. Subjects had an average onset of vision loss of 6 years at the time of treatment. At baseline, both treated (TE) and untreated (UTE) eyes had an off-chart median visual acuity.1
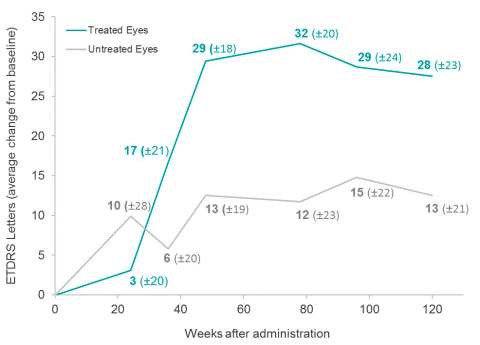
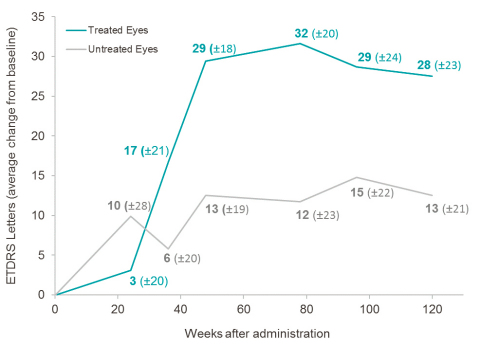
At year 2.5 post-injection, in subjects less than 2 years from onset of vision loss and with relatively better vision at the time of treatment (<2.79 LogMAR),2 TE had a mean gain of +28 ETDRS letters (-0.55 LogMAR) compared to baseline, while UTE had a mean gain of +13 ETDRS letters (-0.25 LogMAR) compared to baseline. The difference of +15 ETDRS letters in favor of TE is clinically significant, and the magnitude of the improvement, which is similar to the trend observed at Weeks 48, 78, and 96, suggests sustained benefit from GS010.
1 At baseline, treated worse-seeing eyes had a median visual
acuity of 2.79 LogMAR (approximately equivalent to hand motion at 1m)
and untreated better-seeing eyes had a median acuity of 2.01 LogMAR
(approximately equivalent to counting fingers at 50cm).
2
2.79 LogMAR is approximately equivalent to hand motion at 1m. Phase III
protocol excludes “hand motion” patients.
The subject group (n=5) with an onset of vision loss of less than 2 years and relatively better vision at the time of injection (<2.79 LogMAR) demonstrated a sustained pharmacological trend in favor of the treated eye, of increasing magnitude from Week 36 onwards, with 60% of subjects showing a clinically significant gain of ≥ 15 letters in TE at year 2.5.3 The characteristics of this subject group are similar to the baseline characteristics of LHON patients enrolled in ongoing Phase III REVERSE and RESCUE clinical trials4.
In all subjects (n=14), the mean change from baseline of visual acuity in TE showed an improvement of +29 ETDRS letters (-0.58 LogMAR) that was both clinically and statistically significant (p = 0.0034), while the mean change in UTE from baseline of visual acuity showed an improvement of +22 ETDRS letters (-0.44 LogMAR), which was not statistically significant. The difference of +7 ETDRS letters was in favor of TE.
At year 2.5 post-injection, GS010 continued to demonstrate a favorable tolerability profile, with no reports of worsening vision or ocular sequelae, serious treatment-emergent adverse events (TEAEs), nor systemic adverse events (AEs) related to GS010 or its administration. Consistent with previously reported data, ocular AEs were mostly mild, well-tolerated, and reversible, responding to standard therapy.
3 Clinical significance is defined as a change from baseline
of at least -0.3 LogMAR (≥ +15 ETDRS letters)
4 One
patient had vision loss for 9 months at treatment administration
(eligible to REVERSE, 6 to 12 months after onset), and 4 patients had a
duration of vision loss > 1 year and < 2 years. None would have been
eligible to RESCUE (< 6 months after onset).
Bernard Gilly, CEO and co-founder of GenSight, commented, “Not only do we see a significant clinical benefit in recently diagnosed subjects, but this benefit is sustained after 2.5 years with a single injection of GS010. This is particularly encouraging, as we are now less than 6 months away from Phase III efficacy data with RESCUE and REVERSE.”
Dr. Catherine Vignal, investigator of the study and Chief of the Department of Ophthalmology at the Rothschild Foundation Hospital in Paris, added, "The confirmatory safety and continued positive trends after 2.5 years of follow-up constitute significant hope for patients suffering from LHON. It is worth noting that the observed improvement in some of the untreated eyes was consistent with several prior studies in neuroretinal degenerative diseases. The insights gained from this and forthcoming data will be tremendously helpful, as GenSight works to develop a therapy for this severe blinding disease affecting patients in the prime of their life, and for which no curative treatment exists.”
GenSight Biologics is currently conducting two Phase III clinical studies (RESCUE and REVERSE) in Europe and the United States to assess the efficacy of GS010 in subjects affected with LHON due to the ND4 mutation, and with vision loss up to one year at the time of treatment. Recruitment of REVERSE was completed in February 2017, and RESCUE was completed in August 2017. Topline results at 48 weeks for REVERSE and RESCUE are expected in the second and third quarters of 2018, respectively.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for neurodegenerative retinal diseases and diseases of the central nervous system. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics for retinitis pigmentosa, to help preserve or restore vision in patients suffering from severe degenerative retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible low vision and legal blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About GS010
GS010 targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research works conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of patients have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously. The estimated incidence of LHON is approximately 1,400 to 1,500 new patients who lose their sight every year in the United States and Europe.
About RESCUE and REVERSE
RESCUE and REVERSE are two separate randomized, double-masked, sham-controlled pivotal Phase III trials designed to evaluate the efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in subjects affected by LHON due to the G11778A mutation in the mitochondrial ND4 gene.
The primary endpoint will measure the difference in efficacy of GS010 in treated eyes compared to sham-treated eyes based on Best Corrected Visual Acuity (BCVA), as measured with the ETDRS at 48 weeks post-injection. The patients’ LogMAR (Logarithm of the Minimal Angle of Resolution) scores, which are derived from the number of letters patients read on the ETDRS chart, will be used for statistical purposes. Both trials have been adequately powered to evaluate a clinically relevant difference of at least 15 ETDRS letters between treated and untreated eyes adjusted to baseline.
The secondary endpoints will involve the application of the primary analysis to best seeing eyes that received GS010 compared to those receiving sham, and to worse seeing eyes that received GS010 compared to those that received sham. Additionally, a categorical evaluation with a responder analysis will be evaluated, including the proportion of patients who maintain vision (< ETDRS 15L loss), the proportion of patients who gain 15 ETDRS letters from baseline and the proportion of patients with Snellen acuity of >20/200. Complementary vision metrics will include automated visual fields, optical coherence tomography, and color and contrast sensitivity, in addition to quality of life scales, bio-dissemination and the time course of immune response.
The trials are conducted in parallel, in 37 subjects for REVERSE and 39 subjects for RESCUE, in 7 centers across the United States, the UK, France, Germany and Italy. Topline results of REVERSE at 48 weeks are expected in Q2 2018, while RESCUE is expected to read out in Q3 2018.
ClinicalTrials.gov Identifiers:
REVERSE: NCT02652780
RESCUE:
NCT02652767