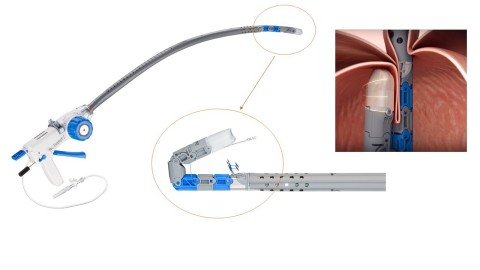
REDMOND, Wash.--(BUSINESS WIRE)--EsophyX® Z+ device offers expanded endoscope compatibility and joins EndoGastric Solutions’® portfolio of specialized technologies used to perform the TIF 2.0 procedure to reconstruct the gastroesophageal valve and restore its function as a reflux barrier.
Using a transoral approach, the EsophyX Z+ device enables physicians to rebuild the gastroesophageal valve with a partial fundoplication technique that secures the tissue with dual deployment of SerosaFuse® Implantable Fasteners.
Michael Murray, M.D., FACS, Northern Nevada Medical Center and Kenneth Chang, M.D., UC Irvine Health were the first to perform the TIF 2.0 procedure using the new EsophyX Z+ device. Both clinicians are well-versed in the TIF 2.0 procedure and have been treating patients with severe gastroesophageal reflux disease (GERD) symptoms for nearly a decade.
“GERD is a chronic condition and proton pump inhibitors do not control symptoms in about one-third of patients,” says surgeon Michael Murray, M.D., Northern Nevada Medical Center. “The design of the new EsophyX Z+ device makes performing TIF 2.0 procedures technically easy and produces a consistent and effective fundoplication thus providing an effective alternative to prescription medications and traditional surgical procedures.”
“The robust collection of clinical data and recent positive policy coverage announcements support the TIF 2.0 procedure as an established treatment option for patients who wish to eliminate or discontinue PPI use and avoid surgery for acid reflux. This newest design iteration allows for maximal visibility using a standard endoscope combined with almost effortless and confident deployment of fasteners. GI endoscopists and surgeons should consider the TIF procedure in patients seeking a less invasive, proven GERD treatment solution,” adds Gastroenterologist Kenneth Chang, M.D., UC Irvine Health.
The original EsophyX device was cleared by the FDA in 2007. EGS launched the third generation EsophyX Z model platform in 2015. The evolving EsophyX Z+ technology now enables surgeons and gastroenterologists to use a wider selection of standard endoscopes to treat the underlying anatomical cause of GERD.
“We are excited to launch the EsophyX Z+ device as we believe it will further enhance the physician’s technical experience,” says Skip Baldino, President and CEO of EndoGastric Solutions. “At EndoGastric Solutions, we are committed to addressing the unmet needs in gastrointestinal diseases and offering physicians highly effective options that fill the significant treatment gap between medication and more invasive surgery.”
About GERD
Gastroesophageal reflux disease (GERD) is a chronic condition in which the gastroesophageal valve (GEV) allows gastric contents to reflux (wash backwards) into the esophagus, causing heartburn and possible injury to the esophageal lining. In the U.S., GERD is the most common gastrointestinal-related diagnosis physicians make during clinical visits. Pain and discomfort from acid reflux impact more than 80 million Americans at least once a month according to estimates. The first treatment recommendations for GERD patients is to make lifestyle changes (e.g., diet, scheduled eating times and sleeping positions). Patients are instructed to take prescription medications; unfortunately, it is a common practice to increase medication doses and, over time, become dependent on these medications to control symptoms. A variety of other health complications are linked to the long-term, maximum-dose usage of prescription medications.
About Transoral Incisionless Fundoplication (TIF®) procedure for reflux
Performed without the need for external incisions through the skin, the TIF procedure offers patients who require an anatomical repair an effective treatment option to correct the underlying cause of GERD. Most patients stopped using daily medications to control symptoms and had their esophageal inflammation (esophagitis) eliminated up to three years after the TIF procedure based on studies.
There are more than 20,000 TIF patients treated worldwide since the EsophyX® device launched. In the past ten years, over 60 centers published more than 80 peer-reviewed papers. These studies document consistent outcomes on over 1,300 unique study patients. For more information, visit www.GERDHelp.com.
About EsophyX® technology
The EsophyX technology is used to reconstruct the gastroesophageal valve (GEV) and restore its function as a barrier, preventing stomach acids refluxing back into the esophagus. The device is inserted through the patient’s mouth with direct visual guidance from an endoscope. The U.S. Food and Drug Administration cleared the original EsophyX device in 2007. EndoGastric Solutions® launched the third generation EsophyX device, the EsophyX Z model, in 2015. The evolving EsophyX technology now enables surgeons and gastroenterologists to use a wider selection of endoscopes to treat the underlying anatomical cause of GERD. These options include low profile and larger high-definition models.
Indications
The EsophyX device with SerosaFuse® fasteners and accessories are indicated for use in transoral tissue approximation, full thickness plication and ligation in the gastrointestinal tract. They are indicated for the treatment of symptomatic chronic GERD in patients who require and respond to pharmacological therapy. The device is also indicated to narrow the gastroesophageal junction, and reduce hiatal hernia ≤ 2cm in size in patients with symptomatic chronic GERD. Patients with hiatal hernias larger than 2cm may be included, when a laparoscopic hiatal hernia repair reduces the hernia to 2cm or less.
About EndoGastric Solutions®
Based in Redmond, WA, EndoGastric Solutions, Inc. (www.endogastricsolutions.com), is a medical device company developing and commercializing innovative, evidence-based, incisionless surgical technology for the treatment of GERD. EGS has combined the most advanced concepts in gastroenterology and surgery to develop the Transoral Incisionless Fundoplication (TIF®) 2.0 procedure—a minimally invasive solution that addresses a significant unmet clinical need. Join the conversation on Twitter: @GERDHelp Facebook: GERDHelp and Google+: GERDHelp.