CAMBRIDGE, England--(BUSINESS WIRE)--AstraZeneca today announced that the New England Journal of Medicine has published the positive results from the Phase III FLAURA trial which provide data for Tagrisso’s (osimertinib) use in the 1st-line treatment of adult patients with locally advanced or metastatic epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC).1 The trial showed a statistically significant, clinically meaningful progression-free survival (PFS) advantage for osimertinib, a third-generation, irreversible EGFR tyrosine kinase inhibitor (TKI), compared with current 1st-line EGFR-TKIs, erlotinib or gefitinib.1
Dr. Suresh S. Ramalingam, Principal Investigator of the FLAURA trial, from the Winship Cancer Institute of Emory University, Atlanta, USA, said: “The results of the FLAURA trial may herald a shift in how we treat patients with EGFR-mutated NSCLC. The data demonstrate superiority of osimertinib compared to current standard EGFR-TKIs in the 1st-line setting.”
Sean Bohen, Executive Vice President, Global Medicines Development and Chief Medical Officer at AstraZeneca, said: “EGFR-mutated NSCLC patients need new therapies that improve outcomes. The data published in NEJM today further emphasise the potential of osimertinib as a new treatment standard in this patient population.”
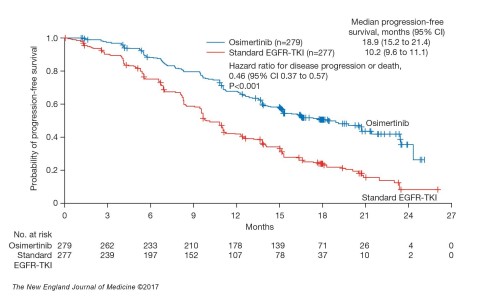
In the Phase III FLAURA trial, osimertinib significantly improved PFS compared to erlotinib or gefitinib in previously untreated patients with locally advanced or metastatic EGFR-mutated (EGFRm) NSCLC. Median PFS was nearly doubled at 18.9 months for osimertinib compared with 10.2 months for the EGFR-TKI comparator arm (PFS, hazard ratio [HR] 0.46; 95% confidence interval [CI] 0.37-0.57; p<0.001). Preliminary overall survival (OS) data favoured osimertinib with a 37% reduction in the risk of death (HR 0.63, 95% CI 0.45-0.88; p=0.007 [not significant]) at the interim OS analysis (25% maturity).1
The FLAURA safety data for osimertinib were in line with those observed in prior clinical trials. Osimertinib was well tolerated, with less frequent grade 3 or higher adverse events (AEs) than with standard EGFR-TKIs (34% vs. 45%). In all patients, the most common AEs were rash or acne (58% [1% Grade ≥3] for osimertinib vs. 78% [7% Grade ≥3] for the comparator arm), diarrhoea (58% [2% Grade ≥3] for osimertinib vs. 57% [2% Grade ≥3] for the comparator arm), and dry skin (36% [<1% Grade ≥3] for osimertinib vs. 36% [1% Grade ≥3] for the comparator arm).1
AstraZeneca presented the full FLAURA data at the Presidential Symposium of the European Society of Medical Oncology (ESMO) 2017 Congress in Madrid, Spain, in September.
NOTES TO EDITORS
About NSCLC
Lung cancer is the leading cause of cancer death
among both men and women, accounting for about one-quarter of all cancer
deaths, more than breast, prostate and colorectal cancers combined.2
Approximately 10-15% of patients in the US and Europe, and 30-40% of
patients in Asia have EGFRm NSCLC.3,4,5 These patients are
particularly sensitive to treatment with currently available EGFR-TKIs,
which block the cell-signaling pathways that drive the growth of tumour
cells.6 However, tumours almost always develop resistance to
EGFR-TKI treatment leading to disease progression.7
Approximately half of patients develop resistance to approved EGFR-TKIs
such as gefitinib and erlotinib due to the resistance mutation, EGFR
T790M. Osimertinib also targets this secondary mutation that leads to
disease progression.7,8 There is also a need for medicines
with improved CNS efficacy, since approximately 25% of patients with
EGFR-mutated NSCLC have brain metastases at diagnosis, increasing to
approximately 40% within two years of diagnosis.9
About Tagrisso
Tagrisso (osimertinib) is
a third-generation, irreversible EGFR-TKI designed to inhibit both
EGFR-sensitising and EGFR T790M-resistance mutations, with clinical
activity against CNS metastases.10 Osimertinib 40mg and 80mg
once-daily oral tablets have been approved in more than 60 countries,
including the US, EU, Japan and China, for patients with EGFR T790M
mutation-positive advanced NSCLC. Osimertinib is also being investigated
in the adjuvant setting and in combination with other treatments.11,12
About FLAURA
The FLAURA trial assessed the efficacy and
safety of osimertinib 80mg once daily vs. standard-of-care EGFR-TKIs
(either erlotinib [150mg orally, once daily] or gefitinib [250mg orally,
once daily]) in previously untreated patients with locally advanced or
metastatic EGFR-mutated NSCLC.13 The trial was a
double-blinded, randomised study, with 556 patients across 29 countries.13
About AstraZeneca in Lung Cancer
AstraZeneca is committed to
developing medicines to help every patient with lung cancer. We have two
approved medicines and a growing pipeline that targets genetic changes
in tumour cells and boosts the power of the immune response against
cancer. Our unrelenting pursuit of science aims to deliver more
breakthrough therapies with the goal of extending and improving the
lives of patients across all stages of disease and lines of therapy.
About AstraZeneca in Oncology
AstraZeneca has a deep-rooted
heritage in Oncology and offers a quickly-growing portfolio of new
medicines that has the potential to transform patients’ lives and the
Company’s future. With at least six new medicines to be launched between
2014 and 2020, and a broad pipeline of small molecules and biologics in
development, we are committed to advance New Oncology as one of
AstraZeneca’s five Growth Platforms focused on lung, ovarian, breast and
blood cancers. In addition to our core capabilities, we actively pursue
innovative partnerships and investments that accelerate the delivery of
our strategy, as illustrated by our investment in Acerta Pharma in
haematology.
By harnessing the power of four scientific platforms – Immuno-Oncology, Tumour Drivers and Resistance, DNA Damage Response and Antibody Drug Conjugates – and by championing the development of personalised combinations, AstraZeneca has the vision to redefine cancer treatment and one day eliminate cancer as a cause of death.
About AstraZeneca
AstraZeneca is a global, science-led
biopharmaceutical company that focuses on the discovery, development and
commercialisation of prescription medicines, primarily for the treatment
of diseases in three therapy areas - Oncology, Cardiovascular &
Metabolic Diseases and Respiratory. The Company also is selectively
active in the areas of autoimmunity, neuroscience, and infection.
AstraZeneca operates in over 100 countries and its innovative medicines
are used by millions of patients worldwide.
For more information, please visit www.astrazeneca.com and follow us on Twitter @AstraZeneca.
Intended audiences
This press release is issued from
AstraZeneca Corporate Headquarters in Cambridge, UK and is intended to
provide information about our global business. Please be aware that
information relating to the approval status and labels of approved
products may vary from country to country, and a country-specific press
release on this topic may have been issued in the countries where
AstraZeneca conducts business.
References
1 Soria, JC, et al. Osimertinib in EGFR Mutation-Positive Advanced Non-Small Cell Lung Cancer. NEJM. To be published 18 Nov 2017.
2 American Cancer Society. Key Statistics for Lung Cancer. Available at https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-statistics.html. Accessed November 2017.
3 Szumera-Ciećkiewicz A, et al. EGFR Mutation Testing on Cytological and Histological Samples in Non-Small Cell Lung Cancer: a Polish, Single Institution Study and Systematic Review of European Incidence. Int J Clin Exp Pathol. 2013:6;2800-12.
4 Keedy VL, et al. American Society of Clinical Oncology Provisional Clinical Opinion: Epidermal Growth Factor Receptor (EGFR) Mutation Testing for Patients with Advanced Non-Small-Cell Lung Cancer Considering First-Line EGFR Tyrosine Kinase Inhibitor Therapy. J Clin Oncol. 2011:29;2121-27.
5 Ellison G, et al. EGFR Mutation Testing in Lung Cancer: a Review of Available Methods and Their Use for Analysis of Tumour Tissue and Cytology Samples. J Clin Pathol. 2013:66;79-89.
6 Langer CJ, et al. Epidermal Growth Factor Receptor Inhibition in Mutation-Positive Non-Small-Cell Lung Cancer: Is Afatinib Better or Simply Newer? J Clin Oncol. 2013:31(27);3303-05.
7 Yu HA, et al. Analysis of Tumour Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancer. Clin Cancer Research. 2013:19(8);2240-46.
8 Wu SG, et al. The Mechanism of Acquired Resistance to Irreversible EGFR Tyrosine Kinase Inhibitor Afatinib in Lung Adenocarcinoma Patients. Oncotarget. 2016:7(11);12404-13.
9 Rangachari, et al. Brain Metastases in Patients with EGFR-Mutated or ALK-Rearranged NonSmall-Cell Lung Cancers. Lung Cancer. 2015;88,108–111
10 Cross DAE, et al. AZD9291, an Irreversible EGFR TKI, Overcomes T790M-Mediated Resistance to EGFR Inhibitors in Lung Cancer. Cancer Discov. 2014:4;1046-61.
11 National Institutes of Health. AZD9291 Versus Placebo in Patients With Stage IB-IIIA Non-small Cell Lung Carcinoma, Following Complete Tumour Resection With or Without Adjuvant Chemotherapy (ADAURA). Available at: https://www.clinicaltrials.gov/ct2/show/NCT02511106. Accessed November 2017.
12 National Institutes of Health. AZD9291 in Combination With Ascending Doses of Novel Therapeutics. Available at: https://clinicaltrials.gov/ct2/show/NCT02143466. Accessed November 2017.
13 Ramalingam S, et al. Osimertinib vs SoC EGFR-TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC (FLAURA). Presented at the European Society for Medical Oncology (ESMO) 2017 Congress, 8-12 September 2017, Madrid, Spain.