ST. PAUL, Minn.--(BUSINESS WIRE)--The Centers for Disease Control (CDC) recently updated its recommendation on use of chlorhexidine-impregnated dressings in its globally-recognized Guidelines for the Prevention of Intravascular Catheter-Related Infections (2011). The new recommendation calls for use of chlorhexidine-impregnated dressings with an FDA-cleared label that specifies a clinical indication for reducing catheter-related bloodstream infection (CRBSI) or catheter-associated bloodstream infection (CABSI) to protect the insertion site of short-term, non-tunneled central venous catheters for patients aged 18 years and older.
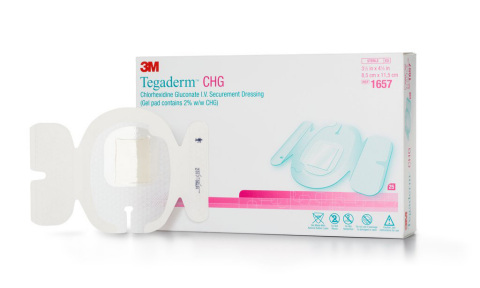
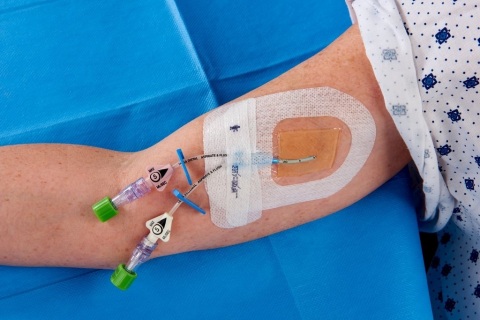
3M Tegaderm CHG I.V. Securement Dressing remains the only transparent dressing indicated and proven to reduce CRBSI, aligning with evidence-based guidelines and practice standards. This includes the 2016 Infusion Therapy Standards of Practice recommendation for the use of transparent dressings that permit continuous visual inspection of the catheter site.1
“The CDC’s evidence-based recommendations elevate current best practices in reducing life-threatening and costly bloodstream infections. The revisions highlight the strong clinical data that supports use of Tegaderm CHG I.V. Securement Dressing worldwide,” said Pat Parks, MD, PhD, medical director for 3M Critical and Chronic Care Solutions Division. “At 3M, our goal is zero bloodstream infections. We’ll keep innovating and educating to make that future possible.”
Tegaderm CHG I.V. Securement Dressing meets the CDC’s highest evidence-based recommendation category, IA, meaning it is a strong recommendation supported by high-to-moderate quality evidence suggesting net clinical benefits or harms. This evidence includes a randomized controlled trial of 1,879 subjects that found Tegaderm CHG I.V. Securement Dressing reduced CRBSI by 60 percent in patients with central and arterial lines (p=0.020).2 The Dressing provides an integrated solution that offers infection reduction, site visibility, consistent application and catheter securement in one easy-to-use product to provide a more convenient and reliable solution for I.V. care and maintenance.
For more information about the clinical evidence supporting Tegaderm CHG I.V. Securement Dressing and how the Dressing meets other industry standards and guidelines, please visit 3M.com/TegadermCHG.
3M and Tegaderm are trademarks of 3M Company.
About 3M
At 3M, we apply science in collaborative ways to
improve lives daily. With $30 billion in sales, our 90,000 employees
connect with customers all around the world. Learn more about 3M’s
creative solutions to the world’s problems at www.3M.com or
on Twitter @3M or @3MNews.
1 Infusion Nurses Society (INS). Infusion Therapy
Standards of Practice. INS; 2016.
2 Timsit JF et al. Randomized
controlled trial of chlorhexidine dressing and highly adhesive dressing
for preventing catheter-related infections in critically ill adults.
Am J Crit Care Med. 2012;186(12): 1272-1278 http://www.atsjournals.org/doi/pdf/10.1164/rccm.201206-1038OC.