FORT WORTH, Texas--(BUSINESS WIRE)--DFB Pharmaceuticals, a private investment and development group, in collaboration with CritiTech and US Biotest, has formed NanOlogy to finance and develop a breakthrough technology platform to produce unique, patented, naked nanoparticle forms of paclitaxel and docetaxel for local delivery with the potential for greater efficacy and safety to treat cancer and other serious illnesses.
NanOlogy has developed sterile suspension forms of NanoPac® (nanoparticle paclitaxel) and NanoDoce® (nanoparticle docetaxel) as well as an inhaled form of NanoPac. A topical form identified as SOR007 (nanoparticle paclitaxel) ointment was developed by affiliate, DFB Soria, and licensed to NanOlogy for clinical development in oncology.
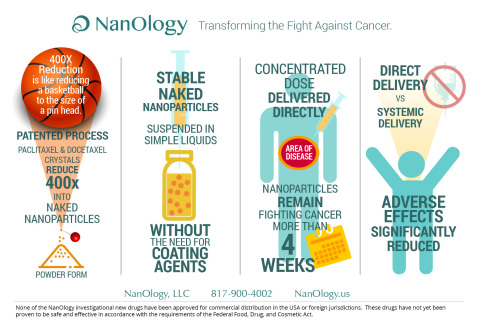
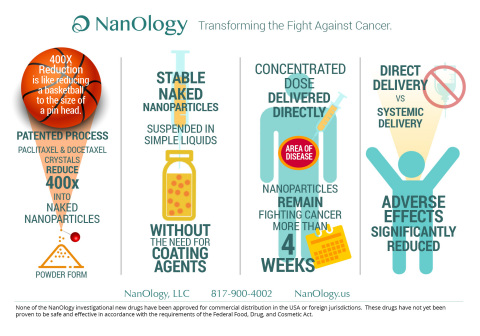
The sterile suspension has been designed to be injected directly into tumors, cysts, peritoneum, or other body cavities, where studies have demonstrated the nanoparticles remain and slowly release for four weeks, resulting in prolonged local exposure. In contrast, systemic forms of taxanes remain at the treatment site for a short time as they are rapidly cleared from the body.
NanOlogy is progressing a broad clinical development program for NanoPac in 2017 that includes clinical trial evaluation of its sterile suspension in ovarian cancer (with orphan drug designation), prostate cancer, pancreatic cancer, and pancreatic mucinous cysts. In addition, clinical trial evaluation of SOR007 ointment is underway for actinic keratosis (under affiliate, Soria), and is expected to begin in the fourth quarter for cutaneous metastases. Clinical trials in various cancers are planned in 2018 for NanoDoce pending approval of its IND, and the inhaled version of NanoPac is in a preclinical efficacy study for lung cancer.
“Systemic administration of paclitaxel and docetaxel is associated with significant adverse effects. Physicians and scientists have known for decades that paclitaxel and docetaxel are effective cancer killing agents, and have long searched for ways to preferentially retain high concentration of drug at the treatment site to increase efficacy,” said Maurie Markman, MD, President of Medicine and Science, Cancer Treatment Centers of America®. “The NanOlogy technology may offer a solution by enabling local delivery of large, sustained amounts of the drug at the site of disease, and reducing systemic exposure and systemic side effects.”
NanoPac and NanoDoce are manufactured by a patented nanoparticle production technology platform that reduces the size of unprocessed paclitaxel and docetaxel crystals by up to 400 times into stable, naked nanoparticles with an exponential increase in surface area and unique geometry. Unlike other nanoparticles, which require coating agents to keep them stable, the patented NanOlogy nanoparticles are stable in their naked form and suspended prior to use in simple vehicles without coating agents.
“We are excited about the potential of this technology platform to bring breakthrough therapies to patients with a wide range of cancers and other serious illnesses,” commented H. Paul Dorman, Chairman and CEO of NanOlogy.
About NanOlogy
NanOlogy, LLC (www.nanology.us) is a company formed by DFB Pharmaceuticals, LLC of Fort Worth, TX, CritiTech, Inc. of Lawrence, KS, and US Biotest, Inc. of San Luis Obispo, CA, to finance and clinically develop a patented nanoparticle technology platform for local, sustained delivery of proven drugs aimed at increasing their safety and efficacy in the treatment of cancer and related conditions.
About DFB Pharmaceuticals
DFB Pharmaceuticals, LLC (www.dfb.com) is a private Texas investment group with an entrepreneurial drive for developing new healthcare products and businesses. Founded in 1990, DFB and its principals have realized more than $1.5 billion in value through startups, strategic acquisition and sale of companies and technologies, internal product development, brand optimization, and operations in the healthcare industry.
About CritiTech
CritiTech, Inc. (www.crititech.com) is private Kansas particle engineering company focused on developing new drugs and improving existing drugs. Using the company’s proprietary Supercritical Precipitation Technology (SCP Technology) CritiTech specializes in optimizing the delivery of challenging drug substances, potent molecules and poorly soluble compounds. In addition, CritiTech uses its SCP Technology to improve the efficacy, drug delivery options, dosing regimen and pharmacokinetics of a wide variety of drugs, including oral, injectable, and inhaled drugs.
About US Biotest
US Biotest, Inc. is a private California company dedicated to the development of therapeutics to address serious unmet medical needs. Building on strong relationships with industry experts, academic institutions, and leading physicians, the company provides product development strategy and support. US Biotest manages efficient delivery of programs from nonclinical through late-stage clinical trials.
Disclaimer
This announcement contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended, including statements about NanOlogy product development, business, and other activities. Such statements are subject to the risks and uncertainties inherent in any pharmaceutical development program which may cause actual results to differ materially due to developmental, clinical trial, regulatory, market, competitive, technological, or other factors. All forward-looking statements are made as of the date of this announcement. DFB disclaims any intent or obligation to update these statements. NanOlogy investigational new drugs have not been approved by FDA for commercial distribution. They have not yet been proven to be safe and effective in accordance with the requirements of the Federal Food, Drug, and Cosmetic Act.