BEDFORD, Mass.--(BUSINESS WIRE)--Augmenix, Inc., a medical technology company that develops, manufactures, and sells proprietary absorbable hydrogels that separate and protect organs at risk during prostate radiotherapy (RT), announced that the International Journal of Radiation Oncology* Biology* Physics (IJROBP), also known as the Red Journal, has published 5-year data on patients treated with SpaceOAR hydrogel prior to RT at the RWTH Aachen University Department of Radiation Oncology, Aachen, Germany.
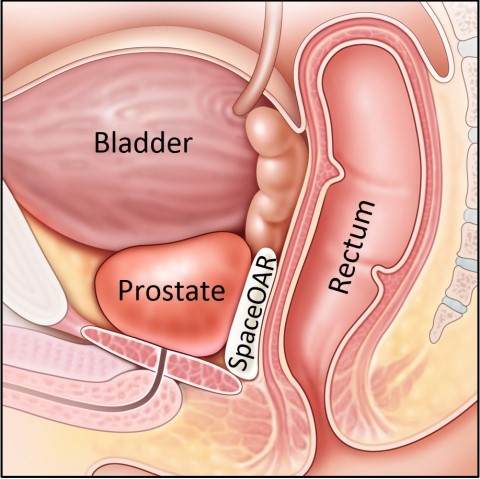
SpaceOAR hydrogel is the only FDA cleared medical device intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and in creating this space it is the intent of SpaceOAR hydrogel to reduce the radiation dose delivered to the anterior rectum.
The publication reports Quality of Life (QOL) outcomes from 114 patients receiving external beam radiotherapy to the prostate with 54 patients receiving SpaceOAR hydrogel prior to RT and 60 patients receiving RT without SpaceOAR hydrogel (Control). The study used the Expanded Prostate Cancer Index Composite (EPIC) survey to document patient QOL at baseline (before RT), at the last day of RT, and at 2, 17 and 63 months following RT.
Significant increases in mean bowel bother QOL scores (>10 points from baseline) were reported over 5X more often by Control patients at 17 months follow up (32% vs. 6%, p<0.01). At 5-year follow-up, 5% of SpaceOAR hydrogel treated patients reported a significantly increased mean bother score vs. 14% of Control patients (p=0.2). SpaceOAR hydrogel treated patients had significantly less moderate to big problems with bowel urgency at 17 months (0 vs 13%, p<0.01) and at 63 months (0 vs 14%, p=0.01), when compared to Control patients. Five Control patients required invasive bowel procedures compared to 1 SpaceOAR hydrogel patient (polypectomy).
A significant finding in terms of sexual QOL was also reported, with SpaceOAR hydrogel treated patients having an 8 times greater likelihood of having erections sufficient for intercourse at 5-years post-treatment (24% vs. 3% p<0.01).
“These long-term results further validate previous 3-year data of the SpaceOAR System and highlight the long-term bowel and sexual Quality of Life benefits it can provide to prostate cancer patients who are treated with radiotherapy,” said Michael Pinkawa, MD, PhD, radiation oncologist at MediClin Robert Janker Klinik, Bonn, Germany and lead author of the study.
“This 5-year data confirms previously reported 3-year outcomes from our randomized, multi-center trial and continues to build a growing portfolio of studies supporting the use of SpaceOAR hydrogel spacing during radiotherapy for prostate cancer,” said John Pedersen, CEO of Augmenix. “We remain committed to furthering the spacing concept that we believe will help many patients return to and maintain pre-treatment quality of life following prostate radiotherapy.”
About SpaceOAR System
Radiation therapy in the treatment of prostate cancer can cause unintended radiation injury to adjacent healthy tissue (organs at risk). This injury can lead to a range of bowel, urinary and sexual symptoms that can affect patient health and quality of life during radiotherapy, and for years afterward. In recent years, radiation oncologists have considered use of “spacing” techniques to reduce the risk of radiation injury to surrounding tissue during radiotherapy. SpaceOAR System is intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and in creating this space it is the intent of SpaceOAR System to reduce the radiation dose delivered to the anterior rectum. The SpaceOAR System is injected as a liquid into the space between the prostate and rectum where it pushes the structures apart and then solidifies into a soft hydrogel. The hydrogel remains stable for three months and then liquefies and is completely absorbed by the body after radiation treatment is over. The SpaceOAR System is FDA cleared and is currently being used in the majority of leading cancer centers in the United States. It is also CE marked, approved in Australia and Japan and licensed in Canada. See the Instructions for Use for complete information on potential risks, warnings and precautions.
About Augmenix, Inc.
Augmenix, Inc. is a privately held company based in the Boston area focused on the development and commercialization of radiation oncology products using its proprietary hydrogel technology. Focusing initially on protection during prostate radiation therapy, the Augmenix next-generation products will address spacing and marking applications throughout the body to improve radiotherapy and interventional oncology procedure outcomes. The company was founded by Incept LLC in 2008 and is funded by several leading venture capital groups. More information about Augmenix and the SpaceOAR System can be found at http://www.Augmenix.com.
1) Hamstra DA, Mariados N, Sylvester J, et al. Continued Benefit to
Rectal Separation for Prostate Radiation Therapy: Final Results of a
Phase III Trial. Int J Radiat Oncol Phys; 2017 Apr 1;97(5):976-985
2)
Hamstra DA, Mariados N, Sylvester J, et al. Sexual Quality of Life
Following Prostate Intensity Modulated Radiotherapy (IMRT) with a
Rectal/Prostate Spacer: Secondary Analysis of a Phase III Trial. Pract
Radiat Oncol. 2017;In Press. doi:10.1016/j.prro.2017.07.008.
SpaceOAR is a registered trademark of Augmenix, Inc.