BEDFORD, Mass.--(BUSINESS WIRE)--Augmenix, Inc., a medical technology company that develops, manufactures, and sells proprietary absorbable hydrogels that separate and protect organs at risk during radiotherapy, today announced that the National Institute for Health and Care Excellence (NICE) in the UK has issued Interventional Procedure Guidance (IPG) supporting the use of a hydrogel barrier to reduce the risk of toxicity to surrounding tissue in radiotherapy to treat prostate cancer. The guidance recommends use of hydrogel spacers under "standard arrangements". For a procedure to be recommended for use with standard arrangements (previously called “normal arrangements”) the evidence should be adequate to show safety and efficacy of the procedure both in the long term and short term. NICE’s guidance clearly indicates that this procedure meets the highest evidence standards.
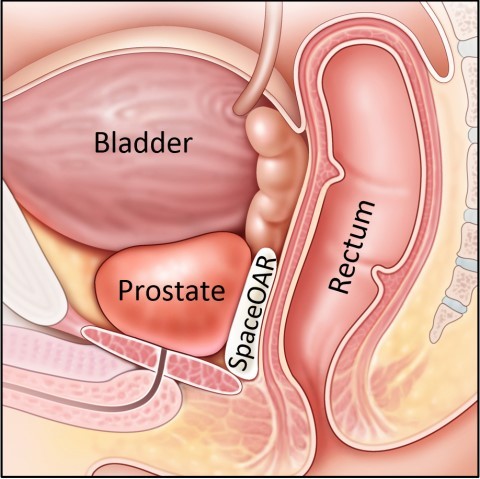
The designation is based in part on 3-year follow-up data from a Phase III prospective, randomized clinical trial involving the use of SpaceOAR hydrogel, an innovative technology developed by Augmenix to reduce the risk of rectal injury in men receiving prostate cancer radiation therapy (RT) by acting as a spacer that pushes the rectum away from the prostate during treatment.
The IPG designation positions UK radiation oncologists and urologists to offer use of a hydrogel spacer such as SpaceOAR as an option for men with prostate cancer who want to reduce their risk of side effects including rectal toxicity, incontinence and loss of sexual function associated with radiotherapy.
“We now have extensive clinical data showing that SpaceOAR hydrogel helps to significantly reduce the risk of rectal and urinary toxicities and loss of sexual function associated with radiation therapy for men with prostate cancer,” said John Pedersen, President & CEO of Augmenix. “We are very pleased that the NICE review and IPG designation were based on data from clinical experience in hundreds of men who have been treated with SpaceOAR hydrogel around the world, and that radiation oncologists and urologists in the UK will be able to offer this proven-effective option to their patients.”
“Radiation therapy has been proven to be a highly effective treatment option for many men with prostate cancer, but in many cases patients are concerned about potential side effects related to rectal toxicity, incontinence or sexual function,” said Professor Heather Payne, a Consultant in Clinical Oncology at University College Hospital, London. “The IPG designation provides important further validation of the benefits of a hydrogel barrier in reducing the risk of side effects, helping patients to proceed to treatment with greater comfort and confidence.”
SpaceOAR® hydrogel reduces rectal injury in men receiving prostate cancer radiation therapy by acting as a spacer – pushing the rectum away from the prostate. This space between organs decreases the radiation dose to the rectum and other Organs at Risk (OAR). Earlier this year, Augmenix announced published data from their prospective, randomized clinical trial showing that patients treated with SpaceOAR hydrogel prior to prostate cancer radiotherapy demonstrated significant rectal (bowel), urinary, and sexual benefits through three years of follow up.
“Our goal in the management of prostate cancer is to identify the most effective treatment options for each patient, and we always have to keep in mind their concerns about side effects that can affect their health and quality of life,” said Dr. Suneil Jain, Senior Lecturer and Consultant in Clinical Oncology at Queen’s University Belfast. “With SpaceOAR Hydrogel, we have a proven safe option to help address some of the most important concerns that patients have about radiotherapy, including bowel toxicity and loss of sexual function.”
About SpaceOAR Hydrogel
Radiation therapy in the treatment of prostate cancer can cause unintended radiation injury to adjacent healthy tissue (organs at risk). This injury can lead to a range of bowel, urinary and sexual symptoms that can affect patient health and quality of life during radiotherapy, and for years afterward. In recent years, radiation oncologists have considered use of “spacing” techniques to reduce the risk of radiation injury to surrounding tissue during radiotherapy. SpaceOAR hydrogel is intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and in creating this space it is the intent of SpaceOAR hydrogel to reduce the radiation dose delivered to the anterior rectum. The SpaceOAR hydrogel is injected as a liquid into the space between the prostate and rectum where it pushes the structures apart and then solidifies into a soft hydrogel. The hydrogel remains stable for three months during radiation therapy then liquefies and is completely absorbed by the body. See the Instructions for Use for complete information on potential risks, warnings and precautions.
About Augmenix, Inc.
Augmenix, Inc. is a privately held U.S. company based in the Boston, Massachusetts area focused on the development and commercialization of radiation oncology products using its proprietary hydrogel technology. Focusing initially on protection during prostate radiation therapy, Augmenix next-generation products will address spacing and marking applications throughout the body to improve radiotherapy and interventional oncology procedure outcomes. The company’s lead product, SpaceOAR System, is FDA cleared and is currently being used in the majority of leading cancer centers in the United States. It is also CE marked, approved in Australia and licensed in Canada. SpaceOAR is a registered trademark of Augmenix, Inc. More information about Augmenix and the SpaceOAR hydrogel can be found at http://www.Augmenix.com.