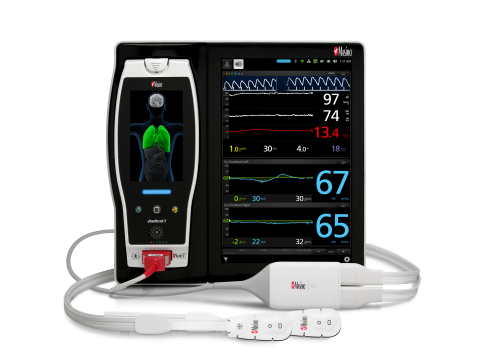
NEUCHATEL, Switzerland--(BUSINESS WIRE)--Masimo (NASDAQ: MASI) announced today the CE marking for the pediatric indication for O3™ regional oximetry with the O3 pediatric sensor. Regional oximetry, also referred to as tissue or cerebral oximetry, helps clinicians monitor cerebral oxygenation.
O3 regional oximetry uses near-infrared spectroscopy (NIRS) to continuously monitor absolute and trended regional tissue oxygen saturation (rSO2) in the cerebral region. Early detection and correction of imbalances in oxygen delivery to the brain and vital organs are important tools in helping patients avoid postoperative morbidity and adverse outcomes.1 With the release of the O3 pediatric sensor, O3 regional oximetry monitoring of rSO2 is now available to pediatric patients weighing less than 40 kg (88 lbs).
“O3 regional oximetry provides access to valuable data about cerebral oxygen saturation, and studies have shown that the risks of cerebral desaturations include neurological injury2,3, increased length of hospital stays3, increased time on mechanical ventilation4, and other adverse outcomes5,” said Joe Kiani, Founder and CEO of Masimo. “With adult trend accuracy of 3% and absolute accuracy of 4% without controlling CO2, and trend accuracy of 3% in pediatric patients6, Masimo O3 should help clinicians build a better picture of brain oxygenation – and hopefully better outcomes for all of their patients, including pediatric ones.”
Masimo O3 regional oximetry and SedLine® brain function monitoring are both available on a single platform, Masimo Root® – opening up a path to better understanding of the brain.
O3 regional oximetry for use with adults weighing 40 kg (88 lbs) or greater has received FDA 510(k) clearance. O3 regional oximetry for use with pediatric patients weighing less than 40 kg (88 lbs) has not received FDA 510(k) clearance; the O3 pediatric sensor is not currently for sale in the United States.
@MasimoInnovates | #Masimo
References
- Booth, Dukatz, Ausman, and Wider. “Cerebral and somatic venous oximetry in adults and infants.” Surg. Neurol Int. 2010; 1: 75.
- Colak Z, Borojevic M, Bogovic A, Ivancan V, Biocina B, Majeric-Kogler V. Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: a randomized, prospective study. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2015 Mar;47(3):447-54.
- Slater JP, Guarino T, Stack J, et al. Cerebral Oxygen Desaturation Predicts Cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg 2009;87:36-45.
- Goldman S, Sutter F, Ferdinand F, Trace C. Optimizing intraoperative cerebral oxygen delivery using noninvasive cerebral oximetry decreases the incidence of stroke for cardiac surgical patients. The heart surgery forum. 2004;7(5):E376-381.
- Deschamps A, Lambert J, Couture P, et al. Reversal of decreases in cerebral saturation in high-risk cardiac surgery. Journal of cardiothoracic and vascular anesthesia. 2013;27(6):1260-1266.
- Masimo data on file.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVI®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (PI). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at http://www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo O3™ Regional Oximetry. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo O3™ Regional Oximetry, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.