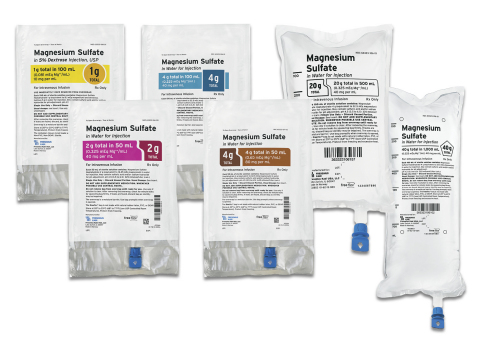
LAKE ZURICH, Ill.--(BUSINESS WIRE)--Fresenius Kabi announced today it has introduced six presentations of Magnesium Sulfate in convenient, ready-to-administer Freeflex® bags. Fresenius Kabi Magnesium Sulfate in Sterile Water for Injection is now available in five Freeflex presentations:
- Magnesium Sulfate 4% Freeflex 50 mL; 2 gm per 50 mL,
- Magnesium Sulfate 4% Freeflex 100 mL; 4 gm per 100 mL,
- Magnesium Sulfate 4% Freeflex 1000 mL; 40 g per 1000 mL,
- Magnesium Sulfate 4% Freeflex 500 mL; 20 g per 500 mL,
- Magnesium Sulfate 8% Freeflex 50 mL; 4 gm per 50 mL.
Additionally, Fresenius Kabi Magnesium Sulfate in 5% Dextrose, USP is now available in a 1 gm per 100 mL presentation.
All six presentations are new offerings from Fresenius Kabi and are available immediately for U.S. customers. The products feature differentiated labeling that can help providers distinguish between different strengths. Fresenius Kabi also offers Magnesium Sulfate in four vial presentations.
Fresenius Kabi is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition.
“These new offerings reflect the growth in demand for our portfolio of convenient, ready-to-administer products,” said John Ducker, president and CEO of Fresenius Kabi USA. “We are pleased to offer the most comprehensive portfolio of Magnesium Sulfate in the United States.”
Magnesium Sulfate is the fifth premix presentation Fresenius Kabi has introduced in the United States since 2013 using the company’s proprietary Freeflex drug delivery system.
Freeflex is a non-PVC and non-DEHP flexible bag for infusion solutions with its patented port technology, clear labeling and innovative product concept.
The Freeflex delivery system has been marketed globally since 2005 and in the U.S. since 2013.
Indications
Magnesium Sulfate is indicated for the prevention and control of seizures in preeclampsia and eclampsia, respectively. Preeclampsia is a syndrome that chiefly includes the development of new-onset high blood pressure in the second half of pregnancy. Eclampsia is the convulsive phase of the disorder and is among the more severe manifestations of the disease.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. For more information about Fresenius Kabi worldwide, please visit www.fresenius-kabi.com.