TORONTO--(BUSINESS WIRE)--FUJIFILM VisualSonics Inc., a world leader in ultra high frequency ultrasound imaging systems and subsidiary of FUJIFILM SonoSite, Inc., today announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Vevo® MD, the world’s first Ultra High Frequency (UHF) clinical ultrasound system. With multiple successes in preclinical research over the last decade, the Vevo® MD is FUJIFILM VisualSonics’ first foray into the clinical market.
“This clearance represents another major accomplishment for FUJIFILM VisualSonics.” said Masayuki Higuchi, president & CEO, FUJIFILM SonoSite, Inc. “This powerful and innovative technology offers unprecedented image resolution capabilities that will have significant impact on the U.S. medical imaging community and the care providers deliver to patients.”
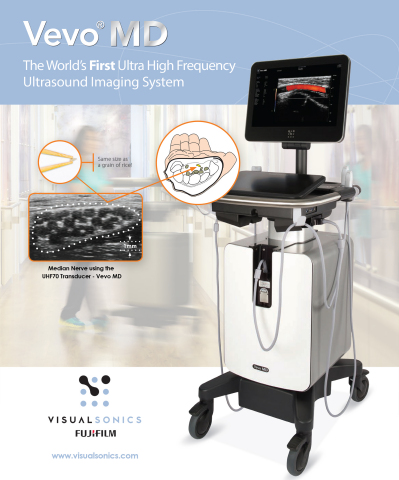
First commercialized in Europe, the Vevo MD is truly a unique ultrasound system, as it operates at much higher frequencies than any conventional ultrasound system currently available. It allows medical professionals to see what they have never seen before—unparalleled image resolution down to 30 micrometers. The Vevo MD system is compatible with FUJIFILM VisualSonics UHF series of transducers. This patented transducer technology is capable of operating in a range of frequencies up to 70 MHz, a tremendous increase in resolution compared to conventional ultrasound systems.
The Vevo MD was designed to play a role in a range of clinical application areas including neonatology, vascular, musculoskeletal, dermatology, and other small parts that are within the first 3 cm of the body.
“As the recognized leader in ultra high frequency imaging systems, FUJIFILM VisualSonics is proud to be the first to market with unparalleled technology.” said Renaud Maloberti, vice president & general manager of FUJIFILM VisualSonics. “With the Vevo MD, clinicians can observe the tiniest, most highly detailed anatomy that has never been seen before, which means greatly enhanced potential for diagnoses.”
“We are confident that Vevo MD is the kind of progressive tool U.S. health care providers will find to be of value for a wide array of applications as well as still unexplored areas.” said Andrew Needles, director of marketing at FUJIFILM VisualSonics. “Our hope is that this innovative new technology will lead to new medical discoveries, and ultimately, improved quality of patient care.”
To learn more about the Vevo MD and upcoming events showcasing this new technology please visit www.vevomd.com or contact FUJIFILM VisualSonics at www.visualsonics.com.
About Fujifilm
FUJIFILM VisualSonics, Inc., is a global leader in real time, in vivo, ultra high frequency ultrasound and photoacoustic imaging systems. With headquarters in Toronto, Canada and offices around the world, FUJIFILM VisualSonics is represented globally across an integrated sales network. FUJIFILM VisualSonics is recognized worldwide for providing cutting edge imaging technologies for the advancement of preclinical research, particularly in cardiovascular, oncology, neurobiology and developmental biology areas. With the expansion of the product portfolio to include a new clinical product, FUJIFILM VisualSonics now broadens their range of imaging technologies across both preclinical and clinical markets. FUJIFILM VisualSonics is a subsidiary of FUJIFILM SonoSite, Inc. and a part of FUJIFILM Holdings Corporation. For more information, please go to: www.visualsonics.com.
FUJIFILM SonoSite, Inc. is the innovator and world leader in bedside and point-of-care ultrasound, and an industry leader in ultra high-frequency micro-ultrasound technology. Headquartered near Seattle, the company is represented by 26 subsidiaries and a global distribution network in over 100 countries. SonoSite’s portable, compact systems are expanding the use of ultrasound across the clinical spectrum by cost-effectively bringing high-performance ultrasound to the point of patient care. For more information, go to: www.sonosite.com.
FUJIFILM Holdings Corporation, Tokyo, Japan brings continuous innovation and leading-edge products to a broad spectrum of industries, including: healthcare, with medical systems, pharmaceuticals and cosmetics; graphic systems; highly functional materials, such as flat panel display materials; optical devices, such as broadcast and cinema lenses; digital imaging; and document products. These are based on a vast portfolio of chemical, mechanical, optical, electronic, software and production technologies. In the year ended March 31, 2015, the company had global revenues of $20.8 billion, at an exchange rate of 120 yen to the dollar. Fujifilm is committed to environmental stewardship and good corporate citizenship. For more information, please visit: www.fujifilmholdings.com.