RAYNHAM, Mass.--(BUSINESS WIRE)--Medrobotics Corp., a surgical robotics company, announced today it has received FDA clearance to market the Flex® Robotic System and is initiating commercial launch in U.S. hospitals. It received European CE mark clearance in March 2014.
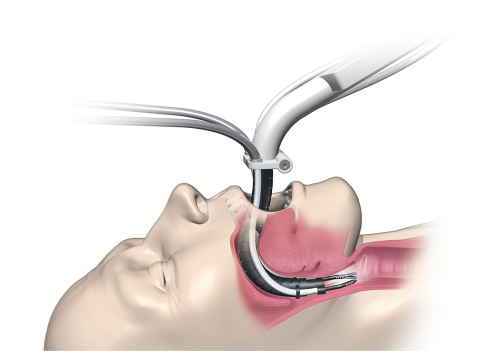
"The Flex® Robotic System is the first and only robot-assisted surgical platform with a flexible scope cleared by FDA for use during transoral procedures," said Samuel Straface, Ph.D., president and CEO of Medrobotics. “The minimally invasive system enables surgical access and visualization in hard-to-reach locations through a single site. Doctors can then complete procedures that might otherwise be difficult, or even impossible, to perform due to inability to visualize or access the site."
Unlike traditional “line-of-sight” approaches, the Flex® Robotic System employs a unique, flexible, robotic scope that precisely moves through the body’s natural twists and turns. Once the surgeon reaches the desired vantage point, the scope becomes rigid to form a stable surgical platform. The on-board, high definition vision system makes it easy to see and operate with a range of flexible surgical instruments. The unique “wristed” 3mm Flex® Instruments allow the surgeon to operate precisely in confined spaces.
"The Flex® Robotic System provides unparalleled access and visualization of the oropharynx, hypopharynx and larynx,” said Dr. Marshall Strome, Professor and Chairman Emeritus, Cleveland Clinic Head and Neck Institute and Co-Chair of Medrobotics Medical Advisory Board. “When used in combination with the highly adjustable Flex® Retractor, surgeons can greatly extend their reach in challenging areas of the mouth and throat. I consider these products transformative.”
University of Pittsburgh Medical Center Distinguished Professor and Emeritus Chair of Otolaryngology, and Co-Chair of Medrobotics Medical Advisory Board, Eugene Myers, M.D., echoed those comments. “I’ve been a surgeon since 1960 and this technology is one of the most significant operating room advances I’ve seen in decades. If surgeons can reach a site, they can do some good. The Flex® Robotic System makes that possible in a number of head and neck procedures. Further, with this device, the surgeon is positioned close to the patient throughout the procedure. My strong sense is it will also have applications for surgery in other sites.”
Minimally invasive surgery has demonstrated advantages for patients and providers compared to traditional open procedures, decreasing hospital stays and recovery times. The Flex® Robotic System was designed to provide an affordable, easy-to-use robot-assisted surgical platform for hospitals and surgeons seeking to provide minimally-invasive treatment options to the broadest number of patients possible.
About Medrobotics
Medrobotics Corporation (www.Medrobotics.com) is a privately-held company headquartered in Raynham, Massachusetts. It manufactures and markets the Flex® Robotic System that was pioneered at Carnegie Mellon University in Pittsburgh, Pennsylvania. This unique, robot-assist surgical platform provides surgeons with single-site access and visualization of hard-to-reach anatomical locations. The Company’s vision is to provide more patients with access to minimally invasive surgery. Medrobotics received the CE mark for its Flex® Robotic System in March 2014. It has been available on a limited basis in Europe since June 2014. The Flex® Robotic System was cleared for sale in the U.S. by the FDA in July 2015.