LOWELL, Mass. & PARIS--(BUSINESS WIRE)--Alcyone Lifesciences, Inc., a leader in neural intervention systems for neurological conditions and targeted drug delivery, and Lysogene, a privately held biotechnology company and leader in recombinant adeno-associated virus (rAAV) based gene therapy for the CNS, have entered into a collaboration. Lysogene and Alcyone Lifesciences will join forces to evaluate the intraparenchymal delivery of Lysogene’s proprietary rAAV to treat patients with mucopolysaccharidosis type IIIA (MPS IIIA), also known as Sanfilippo A, utilizing the Alcyone MEMS Cannula (AMC) targeted delivery platform.
“We are excited about this collaboration. Lysogene is a unique company with incredible science and a strong culture. Karen Aiach and her team are truly impressive. We hope to be part of their success in treating patients with these debilitating conditions,” commented PJ Anand, Founder and Chief Executive Officer of Alcyone Lifesciences.
“Alcyone and Lysogene represent synergy. This is a great opportunity for both companies to forward research in delivering gene therapy to the CNS,” said Karen Aiach, Founder and Chief Executive Officer of Lysogene. “Effective therapeutic and optimized delivery is an absolute requirement for successful therapy. This is an excellent match and I look forward to the outcome of the collaboration,” added Dr. Olivier Danos, Co-founder of Lysogene and Scientific Advisor to Alcyone Lifesciences.
Dr. Michel Zerah, Professor of Neurosurgery at Necker Hôpital Enfants Malades, Paris, welcomed the collaboration and stated, “I look forward to working with Lysogene and Alcyone to further expand our research on effective gene therapy delivery to the brain.”
About Lysogene’s rAAV for MPS IIIA
Lysogene’s most advanced product candidate is rAAV vector serotype rh.10 carrying the human N-sulfoglucosamine sulfohydrolase (hSGSH) for the treatment of MPS IIIA. The recently completed Phase I/II study in four MPS IIIA children demonstrated that the gene therapy and neurosurgical procedure is safe, well tolerated and exploratory efficacy profiles are encouraging (Tardieu 2014).
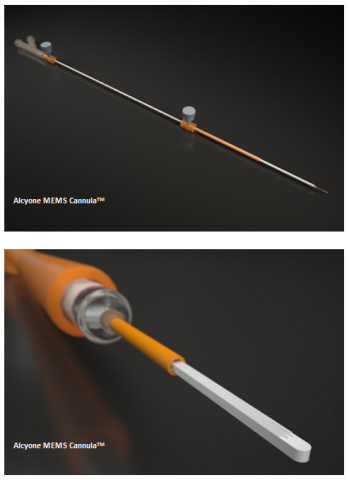
About Alcyone’s MEMS Cannula (AMC) Targeted Delivery System
The Alcyone MEMS Cannula (AMCTM ) Targeted Delivery System was developed using the company’s proprietary microelectromechanical system (MEMS) technology platform. Without burdening the neurosurgery community with unnecessary additional capital equipment, the AMC can be utilized with any existing commercial imaging and stereotactic system in conjunction with the work-flow friendly clinical use guideline designed by the company’s scientist and neurosurgery advisors. Neurosurgeons can select a target, navigate the AMC precisely to the target, and observe in real-time the precision delivery of the therapeutic agent, all under intra-procedural MRI guidance. In addition to the MEMS tip that has dual micro-channels, the AMC features a unique patented distal end design that helps prevent reflux or back flow along the cannula shaft, which can be a significant drawback with current devices. The AMC platform device is designed for optimal targeted bio-distribution and neurosurgeon’s ease of use.
About MPS III A
MPS IIIA presents in early childhood, causing progressive neurodegeneration associated with intractable behavioral problems and developmental regression. Life span is shortened, with death usually occurring in the mid teen years. There is currently no treatment.
Lysogene’s gene therapy is delivered directly to the CNS in one neurosurgical procedure. It is hoped that the delivery of the missing SGSH gene provides a permanent source of functional enzyme in the brain that reverses phenotypic abnormalities of CNS neural cells.
About Lysogene
Lysogene is a global biotechnology company, leader in the basic research and clinical development of gene therapy for neurodegenerative disorders. Its mission is to radically improve the health of patients suffering from incurable life threatening conditions by developing AAV vectors that have demonstrated their effectiveness in safely delivering genetic material to the central nervous system. For more information, please visit www.lysogene.com.
About Alcyone Lifesciences, Inc.
Alcyone Lifesciences, based in Lowell, Massachusetts, is a privately-held medical device company focused on development of novel treatment modalities for chronic neurological conditions. The Company's patented technology platform is based on a uniquely engineered amalgamation of microfabrication technologies along with advanced biomedical engineering with core product focus on targeted drug therapy and hydrocephalus. Alcyone's team of scientists, physicians and advisers includes recognized leaders in the field of neurology and neurosurgery. For more information, please visit www.alcyonels.com.