CHICAGO--(BUSINESS WIRE)--NuCana, a clinical stage biopharmaceutical company developing and commercialising a portfolio of novel anti-cancer medicines, today presented impressive clinical data for its first-in-class, Phase III-ready, anti-cancer agent, Acelarin®, at the 51st Annual Meeting of the American Society of Clinical Oncology (ASCO) in Chicago.
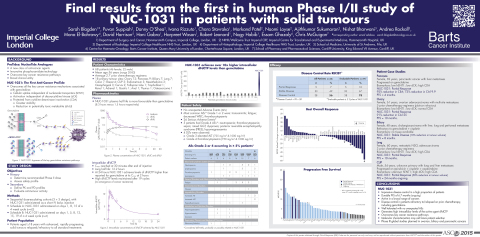
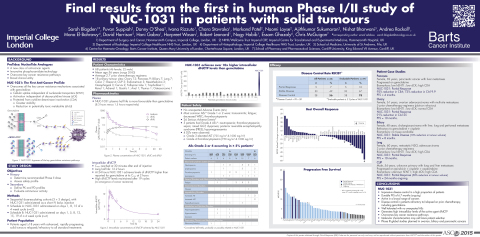
Findings from the first in human study demonstrated that Acelarin (NUC-1031), the first anti-cancer ProTide, achieved a remarkable disease control rate of 78% in patients with advanced, rapidly progressing solid tumours, relapsed or refractory on prior chemotherapy, including gemcitabine. This Phase I/II study reached its primary objective, having established the recommended Phase II dose, and an expansion stage further assessed the efficacy and safety profile. Acelarin was well tolerated with no unexpected adverse events. Clinical activity was observed across a broad range of cancers.
Acelarin is the first in a new class of anti-cancer agents, ProTides, which are specifically designed to overcome key cancer resistance mechanisms that severely limit the action and effectiveness of many commonly prescribed anti-cancer drugs.
The impressive clinical results obtained with Acelarin were presented in the poster session and selected for oral presentation at ASCO’s Poster Highlight Session. Of the forty-nine evaluable patients 10% achieved a Partial Response and 67% Stable Disease, for an overall disease control rate of 78%. This disease control was durable with a progression free survival of 6.7 months ongoing. In addition to the high disease control rate, Acelarin showed a favourable safety profile.
Dr Sarah Blagden, Associate Professor of Experimental Cancer Medicine at Oxford University and Chief Investigator on the Phase I/II study, commented: “The clinical outcomes we achieved with Acelarin are very promising, especially in such a broad range of advanced, relapsed or refractory cancers.”
Hugh Griffith, NuCana’s Chief Executive Officer, said: “Acelarin is the first anti-cancer ProTide to be brought to the clinic and it has shown strong efficacy with a favourable safety profile. This new class of agents has the potential to replace the standard chemotherapy backbone for many patients with cancer. We look forward to commencing our first Phase III study in June.”
A Phase III study comparing Acelarin to gemcitabine for the front line treatment of patients with pancreatic cancer will commence in June 2015. In addition, combination studies with platinums are ongoing for patients with ovarian and biliary cancers.
Poster information at ASCO 2015
Final results from the first in human Phase I/II study of NUC-1031 in patients with solid tumours
Abstract No: 2514
Poster Board Number: Board #230
Time: Saturday May 30, 8:00 AM to 11:30 AM
Location: S Hall A
About NuCana
NuCana® is a rapidly growing, clinical stage biopharmaceutical company with a broad development portfolio of novel anti-cancer medicines. The Company’s proprietary ProTide technology has the potential to set new benchmarks in efficacy and safety with its treatments that are specifically designed to overcome key cancer resistance mechanisms. Acelarin® is NuCana’s lead medicine and was the first ProTide to enter the clinic in October 2010. Acelarin achieved exceptional levels of disease control in a broad range of patients with advanced, rapidly progressing solid tumours. Global Phase III studies with Acelarin are currently being planned in ovarian, biliary and pancreatic cancers. Privately held, NuCana, which raised $57 million in a Series B financing in April 2014, is backed by world-leading investors including Sofinnova Partners, Sofinnova Ventures, Morningside Ventures, Alida Capital International and the Scottish Investment Bank.
For more information, please visit: www.nucana.com
About ProTides
ProTides are first in class pre-activated anti-cancer agents, with a protective phosphoramidate group that allows the medicine to bypass the key tumour resistance mechanisms that limit the activity of many current chemotherapy drugs. Acelarin is the first ProTide in oncology to be brought to the clinic. The innovative ProTide chemistry is a technology platform that can be applied to all nucleoside analogues. Gilead’s ProTide, Sovaldi®, has shown the potential of this new class of medicines for anti-viral therapy.