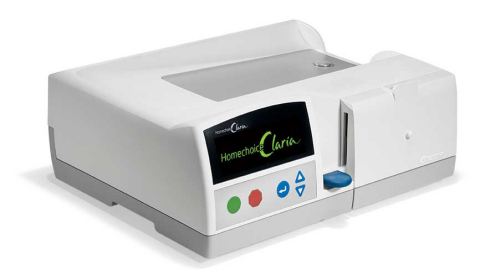
DEERFIELD, Ill.--(BUSINESS WIRE)--Baxter International Inc. (NYSE:BAX) today announced completion of CE marking (market approval) in Europe for HOMECHOICE CLARIA, an automated peritoneal dialysis (APD) system integrated with the SHARESOURCE web-based connectivity platform. The APD system is designed with user-friendly features and secure two-way connectivity so healthcare providers can have increased visibility to monitor their patients’ home peritoneal dialysis (PD) treatments and adjust prescriptions as necessary. PD is a therapy option performed primarily at home by end-stage renal disease patients.
''CLARIA builds upon our market-leading HOMECHOICE APD device with new features to make the system more intuitive for patients to operate in their home and while traveling,'' said Bruce Culleton, M.D., vice president, renal therapeutic area lead, Baxter. ''Additionally, SHARESOURCE, the system’s web-based platform, provides physicians remote access to their home patients’ treatment information allowing for more timely and personalized care.''
HOMECHOICE CLARIA with SHARESOURCE is also designed to save clinics time and improve healthcare practice efficiencies by reducing manual input of data and gathering both patient and clinic data into organized patient report dashboards that are easily accessed and viewed by healthcare providers. In addition, the HOMECHOICE CLARIA system features a large, two-line display screen for better visibility, and a universal interface that is available in 41 languages.
Baxter plans to initiate the commercial launch of HOMECHOICE CLARIA with SHARESOURCE in select European and Asian countries in the first half of 2015.
''Every patient with end-stage renal disease deserves access to individualized care,'' said Jill Schaaf, president of Baxter’s Renal business. ''HOMECHOICE CLARIA with SHARESOURCE represents Baxter’s continued commitment to advancing renal technology that offers healthcare providers and patients therapy options for end-stage renal disease.''
For prescription only. For safe and proper use of the devices mentioned herein, refer to the complete instructions in the Operator's Manual.
Baxter International Inc., through its subsidiaries, develops, manufactures and markets products that save and sustain the lives of people with hemophilia, immune disorders, cancer, infectious diseases, kidney disease, trauma and other chronic and acute medical conditions. As a global, diversified healthcare company, Baxter applies a unique combination of expertise in medical devices, pharmaceuticals and biotechnology to create products that advance patient care worldwide.
This release includes forward-looking statements concerning Baxter’s HOMECHOICE CLARIA automated peritoneal dialysis system, including expectations with regard to future commercial launch plans. The statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those in the forward-looking statements: satisfaction of regulatory and other requirements; actions of regulatory bodies and other governmental authorities; changes in laws and regulations; issues with product quality, manufacturing or supply issues, or patient safety issues; and other risks identified in Baxter's most recent filing on Form 10-K and other SEC filings, all of which are available on Baxter's website. Baxter does not undertake to update its forward-looking statements.