DUBLIN, Ireland--(BUSINESS WIRE)--Research presented today at the International Stroke Conference (ISC) in Nashville, Tenn. and published online in The New England Journal of Medicine (NEJM) found that the addition of the Solitaire™ Device stent thrombectomy procedure to current pharmaceutical treatment significantly reduced disability in patients suffering stroke.
Both the EXtending the Time for Thrombolysis in Emergency Neurological Deficits - Intra-Arterial (EXTEND-IA) and Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) trials, which were investigator initiated trials supported by an unrestricted grant from Medtronic plc (NYSE: MDT), primarily assessed the addition of Solitaire in conjunction with current pharmaceutical treatment, or IV-tPA. ESCAPE assessed 316 patients and found that patients in the treatment arm had a higher rate of return to functional independence (or reduction in stroke morbidity/disability) and lower risk of death from stroke compared to traditional treatment with IV-tPA alone. Functional independence was statistically significantly improved (53% vs 29%) with 53% of patients returned to functional independence after treatment. ESCAPE showed a statistically significant reduction in death (mortality) from stroke, reducing the death rate nearly in half (10% vs. 19%).
Similarly, EXTEND-IA (70 patients) showed a statistically significant improvement in the rate of return to functional independence (71% vs. 40%) in favor of patients treated with Solitaire, when compared to treatment with IV-tPA alone.
EXTEND-IA showed a strong, but not statistically significant, trend towards reduction in death from stroke (9% in the Solitaire arm vs. 20% in the IV-tPA alone arm). In addition, patients treated with Solitaire spent statistically significantly less time in the hospital or in rehabilitation before returning home/to work (15 vs. 73 days). The study realized a statistically significant improvement in recanalization rates at 24 hours of 100% with Solitaire vs. 37% with IV-tPA alone.
“Solitaire stent thrombectomy is the real deal. It represents a sea-change in our treatment of acute ischemic stroke,” said Jeffrey L. Saver, MD, FAHA, FAAN, FANA, professor of Neurology, Geffen School of Medicine at UCLA and director, UCLA Comprehensive Stroke Center.
ESCAPE and EXTEND-IA have now confirmed the findings of the Multi center Randomized Clinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands (MR CLEAN) study, published in NEJM in December 2014. The MR CLEAN results also showed that the addition of stent thrombectomy during early treatment of acute ischemic stroke (AIS) doubled the likelihood of a good neurological outcome.
“The landmark results of ESCAPE and EXTEND-IA indicate that a profound change in how we provide stroke care is needed,” said Bruce Campbell, BMedSc, MBBS (Hons), FRACP, PhD, University of Melbourne. “Our findings provide overwhelming evidence of the safety and efficacy of stent thrombectomy.”
Both ESCAPE and EXTEND-IA were stopped early by the safety committee due to the strength of the results, indicating the benefit of stent thrombectomy with Solitaire. Designed for use to restore blood flow to the brain of patients with ischemic stroke, the Solitaire Device provides an alternative treatment to patients. Many patients are ineligible for IV-tPA either because they have missed the zero to three hour window for treatment to commence, or because they experience a large vessel occlusion or AIS—the most severe stroke— in which case blood clots are too large to dissolve with pharmaceutical treatment, making stent thrombectomy the most viable treatment option.
"We now have an irrefutable body of clinical evidence with a broad patient base supporting the use of endovascular treatment, particularly when stent thrombectomy is used. Guidelines will change," said Michael Hill, BSc, MSc, MD, FRCPC, University of Calgary.
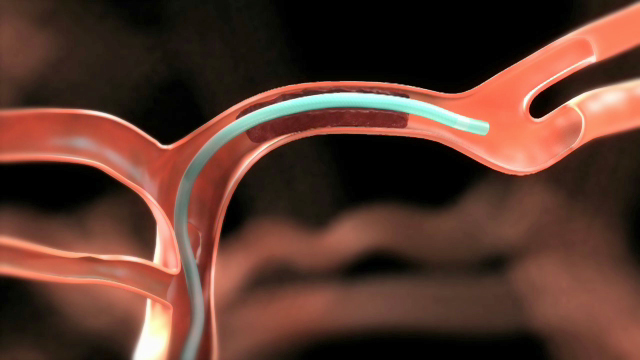
EXTEND-IA and ESCAPE show the effects of Medtronic’s Solitaire Device at restoring blood flow to the brain, reducing stroke morbidity and disability, and reducing the time patients spend in the hospital or rehabilitation center. Both studies met the safety endpoint as well. The Solitaire Device uses a micro-sized catheter to access arteries in the brain affected by stroke through an incision in the leg. Once delivered, the Solitaire Device helps to immediately restore blood flow and remove the blood clots causing the stroke.
“The strength of the data for Solitaire speaks for itself. The data presented today suggest remarkable benefits from stent thrombectomy,” said Brett Wall, president, Neurovascular, Medtronic. “The need for this device in the treatment of stroke is clear.”
According to the World Heart Federation, stroke is the fifth leading cause of death worldwide and contributes to nearly six million deaths around the globe.
About Medtronic
Medtronic plc (www.medtronic.com), headquartered in Dublin, Ireland, is the global leader in medical technology – alleviating pain, restoring health and extending life for millions of people around the world.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.