SAN DIEGO--(BUSINESS WIRE)--For media outside of the US, the UK & Canada only
Boehringer Ingelheim today announced the first Phase III data for the once-daily fixed-dose combination of tiotropium and olodaterol. Results from the Phase III VIVACITO™ study show that once-daily tiotropium + olodaterol FDC improved lung function (FEV1*) of COPD patients to levels significantly above those achieved with tiotropium or olodaterol monotherapies, or with placebo.
The 6-week trial with an incomplete crossover design was conducted in patients with moderate to severe COPD. The results were presented at the American Thoracic Society (ATS) 2014 International Congress in San Diego. Further data from the Phase III programme for tiotropium + olodaterol FDC are due to report later this year.
They are expected to further show the efficacy and safety of the tiotropium + olodaterol FDC and how its benefits in lung function translate into meaningful benefits to patients in their daily lives.
“Many COPD patients remain symptomatic despite current standard care and therefore are limited in their daily activities, like meeting friends or doing outdoor activities. New, innovative treatments are needed to help improve patients’ lung function and their quality of life," said Klaus F. Rabe, Professor of Pulmonary Medicine at the University of Kiel and Director of the Department of Pneumology at Clinic Grosshansdorf, Germany. “The increase of more than 200 ml in trough FEV1 seen in the VIVACITO™ study with tiotropium + olodaterol FDC compared to placebo is significant. A few years ago we would simply not have thought this level of improvement would be possible.”
Once-daily tiotropium + olodaterol FDC is an investigational treatment that contains the well-established once-daily LAMA§ tiotropium (Spiriva®) combined with olodaterol (Striverdi®), the new once-daily and fast-acting LABA** delivered by the Respimat® SoftMist™ Inhaler. Respimat® is an innovative inhaler delivering a unique slow-moving SoftMist™ that allows gentle inhalation – making it easy for patients to take their therapy.
The VIVACITO™ data show that the tiotropium + olodaterol FDC demonstrated clear and consistent improvements in lung function (FEV1*) over 24 hours compared with tiotropium or olodaterol monotherapies, and placebo. In the study, tiotropium + olodaterol FDC was shown to have a safety profile similar to tiotropium.
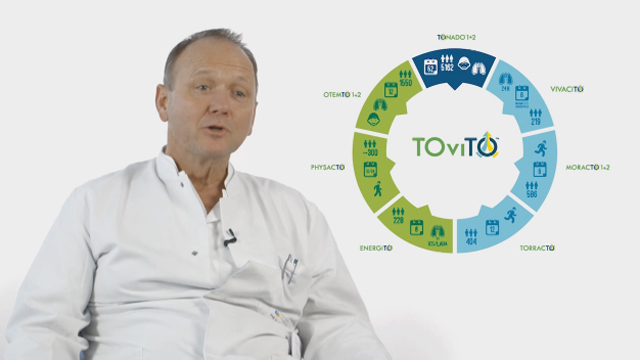
The results from VIVACITO™ are the first Phase III data for tiotropium + olodaterol FDC presented from the TOviTO™ programme, one of the largest Phase III clinical trial programmes ever conducted in COPD. In addition to evaluating the effects of the tiotropium + olodaterol FDC on lung function, the TOviTO™ programme is also focused on the evaluation of other important clinical outcomes related to the daily life of patients with COPD.
Results from the pivotal 52-week Phase III TONADO™ 1&2 trials, investigating the effect of tiotropium + olodaterol FDC on lung function and quality of life in patients with COPD, are due to report later in 2014.
These data will form a major part of Boehringer Ingelheim’s submission to regulatory authorities to support the registration of tiotropium + olodaterol FDC in COPD.
* FEV1 is the maximum volume of air that can be forcibly expired in 1st second, following maximal inspiration and is an important indicator of lung function
†Tiotropium + olodaterol FDC is an investigational treatment. It has not been approved for clinical use. Its safety and efficacy have not yet been fully established
‡Chronic obstructive pulmonary disease
§Long-acting muscarinic antagonist
**Long-acting beta2-agonist
For more detailed information please follow the links below:
- Tiotropium + olodaterol FDC backgrounder: click here
- VIVACITO™ clinical trial backgrounder: click here
- TOviTO™ clinical trial programme backgrounder: click here
- Notes to editors and references: click here