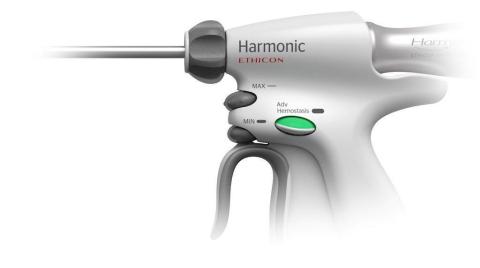
HOLLYWOOD, Fla.--(BUSINESS WIRE)--Ethicon Endo-Surgery, Inc. (Ethicon) today announces the launch of HARMONIC ACE®+7 Shears with Advanced Hemostasis (HARMONIC ACE®+7), the first purely ultrasonic device with a 7 mm sealing indication. HARMONIC ACE®+7 combines the precision and multi-functionality expected of HARMONIC® with reliable large vessel sealing; a benefit combination that does not exist in any other product, representing a true breakthrough in ultrasonic energy technology.
The new device will be featured at The American Society of Colon and Rectal Surgeons (ASCRS) Annual Scientific Meeting in Hollywood, FL this week.
The HARMONIC ACE®+7 is setting a new standard in ultrasonic energy by demonstrating larger vessel sealing strength using the Advanced Hemostasis mode. Benchtop testing shows:
- Greater 5 to 7 mm vessel-sealing reliability than LigaSureTM devices1
- 140% higher median burst pressure vs LigaSureTM 5 mm Blunt Tip, when sealing 5 to 7 mm vessels in the Advanced Hemostasis mode2
- 112% higher median burst pressure vs LigaSure Advance™, when sealing 5 to 7 mm vessels in the Advanced Hemostasis mode3
“The addition of the 7 mm vessel sealing capability to the HARMONIC ACE®+7 makes it an optimal tool for laparoscopic colon surgery. It provides the same precise dissection that I’ve become accustomed to from the HARMONIC ACE® and now with larger vessel sealing capability,” said Bartley Pickron, MD, Colon and Rectal Surgeon, Colorectal Surgical Associates, Houston, Texas. “For many years, surgeons have had to choose between precision and sealing strength. With the HARMONIC ACE®+7, we will no longer need to trade off precision and multi-functionality for sealing strength. Furthermore, the avoidance of using multiple instruments should result in a cost savings for the overall procedure.”
HARMONIC ACE®+7 is designed for use in numerous procedures and specialties including General, Colorectal, Bariatric, Gynecology, Thoracic, and Urology, enhancing surgeons’ ability to handle multiple jobs with superior precision. The new HARMONIC ACE®+7 is best suited for cases which require dissection, mobilization and large vessel sealing.
“The HARMONIC ACE®+7 represents a breakthrough in ultrasonic technology that now enhances surgeon choice. Surgeons no longer need to trade off precision and multi-functionality for sealing strength. It may in some cases also reduce the need for additional devices, such as clips, improving procedure efficiency,” said Tom O’Brien, Ethicon Vice President, Energy Global Strategic Marketing. “Our goal is to continue bringing to market a strong mix of both meaningful innovation (like HARMONIC ACE®+7) and value solutions (like our new Performance Certified HARMONIC® Program) that we believe will change the conversation in clinical practice from an innovation 'or' value conversation to an innovation 'and' value conversation which could ultimately lead to improved outcomes and reduced healthcare costs.”
About Our Energy Business
Ethicon is a leader in advanced energy solutions and offers the broadest portfolio of ultrasonic energy devices using HARMONIC® technology and advanced bipolar energy devices using ENSEAL® technology. Click here to learn more.
HARMONIC® ultrasonic devices combine precision and multifunctionality: Uniquely designed for precise dissection, sealing and transection, one device enables surgeons to perform multiple jobs without instrument exchanges. HARMONIC® technology is the proven leader in advanced energy with more than 17 million procedures worldwide.4 Devices in the HARMONIC® portfolio are used for open and laparoscopic procedures that require unmatched precision. The portfolio can be used across a range of surgical specialties: General, Colorectal, Bariatric, Vascular, Gynecology, ENT, Urology, Thoracic, and Plastic and Reconstructive. Click here to learn more.
ENSEAL® Tissue Sealers consistently cut and seal vessels up to and including 7 mm through high uniform compression. Devices in the ENSEAL® portfolio are intended for use during both open and laparoscopic surgical procedures where division of vessels, lymphatics and tissue bundles is performed. These surgical specialties include: General, Colorectal, Bariatric, Gynecology, Thoracic, Plastic and Reconstructive, Urology, and ENT. Click here to learn more.
About Our Company
Ethicon, Inc. and Ethicon Endo-Surgery, Inc., two companies with long histories of medical innovation, do business under the Ethicon brand. Their surgical technologies and products (including sutures, staplers, energy devices, clip appliers, trocars and meshes) are used around the world to treat colorectal and thoracic conditions, women’s health conditions, hernias, cancer and obesity. Ethicon, Inc. and Ethicon Endo-Surgery, Inc. are part of the Johnson & Johnson Family of Companies. Learn more at www.ethicon.com, and follow us on Twitter @Ethicon.
Dr. Bartley Pickron, MD, is a paid consultant for Ethicon.
1In benchtop test on 5‐7mm porcine carotids that compared burst pressure failures under 240 mmHg, HARMONIC ACE®+7 (2/152 failures) versus LigaSure™ 5mm Blunt Tip and LigaSure™ Advance (15/154 failures) (P = 0.001) Data on file (PRC064872).
2In benchtop test using 5‐7mm porcine carotids that compared median burst pressure for HARMONIC ACE®+7 (1419 mmHg) and LigaSure™ 5mm Blunt Tip (591 mmHg) (p< 0.001). Data on file (PRC064872).
3In benchtop test using 5‐7mm porcine carotids that compared median burst pressure for HARMONIC ACE®+7 (1419 mmHg) and LigaSure™ Advance (670 mmHg) (p< 0.001). Data on file (PRC064872).
4Internal sales data as of June 10, 2013
This press release contains "forward-looking statements" as defined in the Private Securities Litigation Reform Act of 1995. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Ethicon Endo-Surgery, Inc. and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approvals; challenges to patents; economic factors, such as interest rate and currency exchange rate fluctuations; changes in behavior and spending patterns or financial distress of purchasers of health care products and services; changes to governmental laws and regulations and domestic and foreign health care reforms; general industry conditions, including trends toward health care cost containment; and increased scrutiny of the health care industry by government agencies. A further list and description of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended December 29, 2013, including in Exhibit 99 thereto, and our subsequent filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Neither Ethicon Endo-Surgery, Inc. nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.