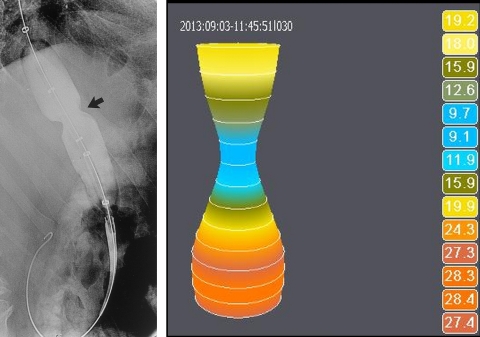
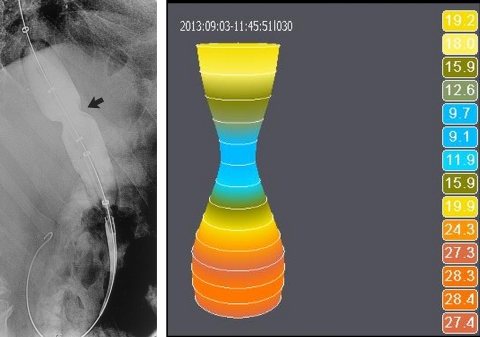
GALWAY, Ireland--(BUSINESS WIRE)--Crospon has today announced that it has received FDA clearance for its EsoFLIP® balloon dilation catheter. This is the first product the in what will be a suite of dilation catheter products to be used for dilation procedures in the esophagus and colon. It represents the company’s first foray into the therapeutic endoscopy accessory market.
Commenting on the FDA clearance, John O’Dea, CEO, Crospon said, “This product marks our entry into an adjacent, significant, established and reimbursed market segment. Whereas up to now our EndoFLIP® product has been primarily used as a measurement device to provide guidance during surgical procedures, EsoFLIP® represents our first therapeutic product, one which will be used during endoscopy. We believe this product can be a game changer in balloon dilation. A unique advantage of EsoFLIP® dilation catheter, versus competitive products, lies in the fact that it eliminates the need for patients to be exposed to radiation during dilation procedures, since the balloon allows measurements of lumen diameter to be made electrically. This will be particularly advantageous for pediatric patients. Equally, from an occupational safety standpoint, staff who perform many of these procedures each day, will no longer be required to be exposed to radiation. Furthermore if a stent is required, these precise measurements assist gastroenterologists, for the first time, in selecting the stent size without the need for radiology”.
Crospon expects to commence first shipments of the EsoFLIP catheter in November 2013.
Ends.
About Crospon
Founded in 2006, and based in Galway, Ireland, Crospon develops leading edge minimally invasive medical devices for monitoring, diagnosis and therapy in the areas of general surgery and gastroenterology. In 2012 the company received the European Enabling Technology Award for Surgical Imaging Technology. CEO John O’Dea is the current President of Engineers Ireland.