FORT WORTH, Texas--(BUSINESS WIRE)--Galderma Laboratories, L.P. today announced that Mirvaso Gel is now available in pharmacies in the U.S. for the treatment of the persistent (nontransient) facial erythema (redness) of rosacea. Mirvaso Gel is the first and only FDA-approved topical prescription treatment indicated for the persistent facial redness of rosacea.
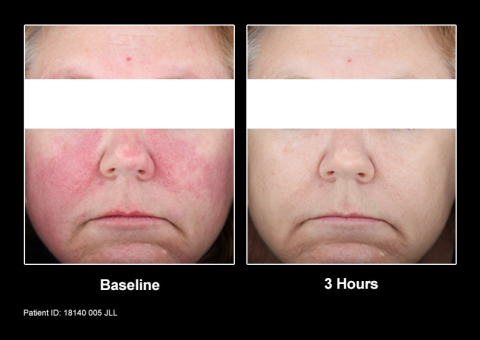
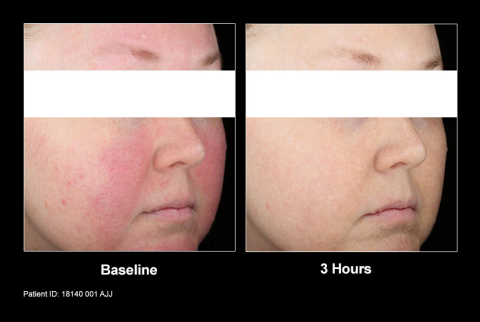
“Mirvaso Gel is an important breakthrough to address the unmet need of treating the persistent facial redness of rosacea,” said Mark Jackson, M.D., Clinical Professor of Medicine at the University of Louisville, dermatologist and a principal investigator for the phase 3 studies of Mirvaso Gel. “Rosacea patients now have an option that works quickly and lasts up to 12 hours to control the redness of rosacea, which is the most common and can be perceived as the most bothersome symptom of the chronic skin condition.”
Mirvaso Gel is a topical gel that may work by constricting the dilated facial blood vessels to reduce the redness of rosacea. The approval of Mirvaso Gel was based on data collected from more than 550 patients enrolled in two phase 3 clinical studies of one-month duration. The results from both studies showed that adults who used Mirvaso Gel demonstrated significantly greater improvement in the facial redness of rosacea than vehicle gel. In addition, a long-term study in 276 subjects who used Mirvaso Gel for up to 12 months was also conducted.
“With the introduction of Mirvaso Gel to the U.S. market, we realize how important it is to treat the persistent facial redness of rosacea, as well as the high level of needs of patients. Physicians are excited about this innovation and also understand it gives them a unique opportunity to reinforce their relationship with patients,” said François Fournier, President of North American Operations, Galderma Laboratories. “Mirvaso Gel is a strong example of our commitment to investing in skin research and delivering innovative medical solutions to address unmet dermatological needs.”
Rosacea is a chronic, inflammatory skin condition that affects an estimated 16 million Americans and 45 million people worldwide. It is characterized by redness, visible facial blood vessels, pimple-like bumps and blemishes that typically appear in the middle of the face (forehead, nose, cheeks) after age 30 in men and women. While treatments exist for the inflammatory bumps and blemishes of rosacea, Mirvaso Gel is the first and only treatment specifically developed and indicated to treat the persistent facial redness of rosacea, which is the most common symptom of the condition. Rosacea can be triggered by spicy foods, alcohol, emotional stress, sun exposure, and hot baths. Because of the physical manifestation of rosacea on the face, the condition can cause embarrassment, anxiety and frustration and can have a negative impact on the patients’ social life. If left untreated, rosacea may worsen. If people suspect that they might have rosacea, they should visit their dermatologist or healthcare provider for diagnosis and treatment.
For more information about this new treatment, visit www.mirvaso.com.
Important Safety Information - Mirvaso® Gel
Indication: Mirvaso® (brimonidine) Topical Gel, 0.33%* is an alpha adrenergic agonist indicated for the topical treatment of persistent (nontransient) facial erythema of rosacea in adults 18 years of age or older. Adverse Events: In clinical trials, the most common adverse reactions (≥1%) included erythema, flushing, skin burning sensation and contact dermatitis. Warnings/Precautions: Mirvaso Gel should be used with caution in patients with depression, cerebral or coronary insufficiency, Raynaud's phenomenon, orthostatic hypotension, thromboangiitis obliterans, scleroderma, or Sjögren’s syndrome. Alpha-2 adrenergic agents can lower blood pressure. Mirvaso Gel should be used with caution in patients with severe or unstable or uncontrolled cardiovascular disease. Serious adverse reactions following accidental ingestion of Mirvaso Gel by children have been reported. Keep Mirvaso Gel out of reach of children. Not for oral, ophthalmic, or intravaginal use.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
________________________
*Each gram of gel contains 5 mg of brimonidine tartrate, equivalent to 3.3 mg of brimonidine free base